A practical strategy to subcutaneous administered in-situ gelling co-delivery system of arsenic and retinoic acid for the treatment of acute promyelocytic leukemia
- PMID: 34849168
- PMCID: PMC8609443
- DOI: 10.1016/j.ajps.2021.07.003
A practical strategy to subcutaneous administered in-situ gelling co-delivery system of arsenic and retinoic acid for the treatment of acute promyelocytic leukemia
Abstract
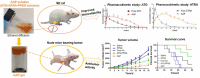
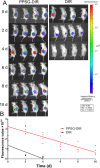
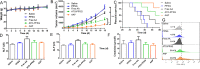
Arsenic trioxide (ATO) combined with all trans retinoic acid (ATRA) is the first choice for the treatment of low and medium risk acute promyelocytic leukemia (APL). Clinical studies reported that the combination of ATO and ATRA could achieve a significant curative effect. However, the retinoic acid syndrome, serious drug resistance and the short half-life in vivo which lead to frequent and large dose administration limit the application of ATRA. In addition, the preparations of arsenic are conventional injections and tablets in clinic, which has poor patients' compliance caused by frequent long-term administration and serious side effects. In order to overcome the above limitations, a phospholipid phase separation gel (PPSG) loaded with ATO and ATRA was developed. ATO+ATRA-PPSG (AAP), as a biodegradable sustained-release delivery system, was the first achievement of co-delivery of hydrophilic ATO and lipophilic ATRA with high drug loading which is the main problem in the application of nano preparation. The prepared PPSG displayed high safety and biocompatibility. The drug in PPSG was released slowly and continuously in vivo and in vitro for up to 10 d, which could reduce the side effects caused by the fluctuation of blood drug concentration and solve the problem of the long treatment cycle and frequent administration. In vivo pharmacokinetics depicted that PPSG could improve the bioavailability, decrease the peak concentration, and prolong the t1/2 of ATO and ATRA. Particularly, AAP significantly inhibited the tumor volume, extended the survival period of tumor-bearing mice, and promoted the differentiation of APL cells into normal cells. Therefore, ATO+ATRA-PPSG not only could co-load hydrophilic ATO and lipophilic ATRA according to the clinical dosage, but also possessed the sustained-release and long-acting treatment effect which was expected to reduce administration time and ameliorate compliance of patients. Thus, it had great potential for clinical transformation and application.
Keywords: All trans retinoic acid; Arsenic trioxide; Bioavailability; Compliance; Phospholipid phase separation gel; Sustained-release.
© 2021 Shenyang Pharmaceutical University. Published by Elsevier B.V.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
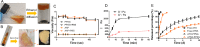


Similar articles
-
Tocotrienols-enriched Self-nanoemulsifying Drug Delivery System Enhances the Antileukemic Activity of All-trans Retinoic Acid but not Electrocardiogram Alterations Evoked by Its Combination with Arsenic Trioxide.AAPS PharmSciTech. 2023 Mar 14;24(3):79. doi: 10.1208/s12249-023-02531-w. AAPS PharmSciTech. 2023. PMID: 36918482
-
Src family kinase inhibitor PP2 enhances differentiation of acute promyelocytic leukemia cell line induced by combination of all-trans-retinoic acid and arsenic trioxide.Leuk Res. 2014 Aug;38(8):977-82. doi: 10.1016/j.leukres.2014.05.019. Epub 2014 Jun 4. Leuk Res. 2014. PMID: 24953245
-
Arsenic trioxide and all-trans retinoic acid (ATRA) treatment for acute promyelocytic leukemia in all risk groups: study protocol for a randomized controlled trial.Trials. 2018 Sep 5;19(1):476. doi: 10.1186/s13063-018-2812-3. Trials. 2018. PMID: 30185214 Free PMC article.
-
[Arsenic trioxide in combination with all-trans retinoic acid for acute promyelocytic leukemia: a systematic review and meta-analysis].Zhong Xi Yi Jie He Xue Bao. 2009 Nov;7(11):1024-34. doi: 10.3736/jcim20091102. Zhong Xi Yi Jie He Xue Bao. 2009. PMID: 19912733 Review. Chinese.
-
Treatment of acute promyelocytic leukaemia with all-trans retinoic acid and arsenic trioxide: a paradigm of synergistic molecular targeting therapy.Philos Trans R Soc Lond B Biol Sci. 2007 Jun 29;362(1482):959-71. doi: 10.1098/rstb.2007.2026. Philos Trans R Soc Lond B Biol Sci. 2007. PMID: 17317642 Free PMC article. Review.
Cited by
-
Preliminary delivery efficiency prediction of nanotherapeutics into crucial cell populations in bone marrow niche.Asian J Pharm Sci. 2023 Nov;18(6):100868. doi: 10.1016/j.ajps.2023.100868. Epub 2023 Nov 19. Asian J Pharm Sci. 2023. PMID: 38089836 Free PMC article.
References
-
- Sanz M.A., Grimwade D., Tallman M.S., Lowenberg B., Fenaux P., Estey E.H., et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113(9):1875–1891. - PubMed
-
- Lo-Coco F., Avvisati G., Vignetti M., Thiede C., Orlando S.M., Iacobelli S., et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

