Physiological and pharmacological modulation of BAX
- PMID: 34848097
- PMCID: PMC8840970
- DOI: 10.1016/j.tips.2021.11.001
Physiological and pharmacological modulation of BAX
Abstract
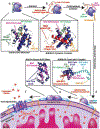
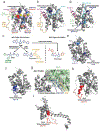
Bcl-2-associated X protein (BAX) is a critical executioner of mitochondrial regulated cell death through its lethal activity of permeabilizing the mitochondrial outer membrane (MOM). While the physiological function of BAX ensures tissue homeostasis, dysregulation of BAX leads to aberrant cell death. Despite BAX being a promising therapeutic target for human diseases, historically the development of drugs has focused on antiapoptotic BCL-2 proteins, due to challenges in elucidating the mechanism of BAX activation and identifying druggable surfaces of BAX. Here, we discuss recent studies that have provided structure-function insights and identified regulatory surfaces that control BAX activation. Moreover, we emphasize the development of small molecule orthosteric, allosteric, and oligomerization modulators that provide novel opportunities for biological investigation and progress towards drugging BAX.
Keywords: BAX; BAX activators; BAX inhibitors; BCL-2 family; apoptosis; mitochondria.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests No interests are declared.
Figures

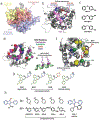
Similar articles
-
Regulation of Bax mitochondrial localization by Bcl-2 and Bcl-x(L): keep your friends close but your enemies closer.Int J Biochem Cell Biol. 2013 Jan;45(1):64-7. doi: 10.1016/j.biocel.2012.09.022. Epub 2012 Oct 11. Int J Biochem Cell Biol. 2013. PMID: 23064052 Review.
-
Small molecules reveal an alternative mechanism of Bax activation.Biochem J. 2016 Apr 15;473(8):1073-83. doi: 10.1042/BCJ20160118. Epub 2016 Feb 25. Biochem J. 2016. PMID: 26916338 Free PMC article.
-
Bid chimeras indicate that most BH3-only proteins can directly activate Bak and Bax, and show no preference for Bak versus Bax.Cell Death Dis. 2015 Apr 23;6(4):e1735. doi: 10.1038/cddis.2015.105. Cell Death Dis. 2015. PMID: 25906158 Free PMC article.
-
Cryo-Electron Microscopy to Study Bax Pores and MOMP.Methods Mol Biol. 2019;1877:247-256. doi: 10.1007/978-1-4939-8861-7_17. Methods Mol Biol. 2019. PMID: 30536011
-
Bax, Bak and beyond - mitochondrial performance in apoptosis.FEBS J. 2018 Feb;285(3):416-431. doi: 10.1111/febs.14186. Epub 2017 Sep 4. FEBS J. 2018. PMID: 28755482 Review.
Cited by
-
Omaveloxolone inhibits IL-1β-induced chondrocyte apoptosis through the Nrf2/ARE and NF-κB signalling pathways in vitro and attenuates osteoarthritis in vivo.Front Pharmacol. 2022 Sep 27;13:952950. doi: 10.3389/fphar.2022.952950. eCollection 2022. Front Pharmacol. 2022. PMID: 36238561 Free PMC article.
-
Value of Bax and Bcl2 expression in peripheral blood mononuclear cells for clinical prognosis of patients with chronic heart failure.Medicine (Baltimore). 2024 Jan 19;103(3):e36943. doi: 10.1097/MD.0000000000036943. Medicine (Baltimore). 2024. PMID: 38241555 Free PMC article.
-
Identification of programmed cell death-related gene signature and associated regulatory axis in cerebral ischemia/reperfusion injury.Front Genet. 2022 Aug 4;13:934154. doi: 10.3389/fgene.2022.934154. eCollection 2022. Front Genet. 2022. PMID: 35991562 Free PMC article.
-
Development of novel cytoprotective small compounds inhibiting mitochondria-dependent cell death.iScience. 2023 Sep 17;26(10):107916. doi: 10.1016/j.isci.2023.107916. eCollection 2023 Oct 20. iScience. 2023. PMID: 37841588 Free PMC article.
-
Isoliquiritigenin alleviates cerebral ischemia-reperfusion injury by reducing oxidative stress and ameliorating mitochondrial dysfunction via activating the Nrf2 pathway.Redox Biol. 2024 Nov;77:103406. doi: 10.1016/j.redox.2024.103406. Epub 2024 Oct 22. Redox Biol. 2024. PMID: 39454290 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

