Modelling the concentration of anti-SARS-CoV-2 immunoglobulin G in intravenous immunoglobulin product batches
- PMID: 34843493
- PMCID: PMC8629175
- DOI: 10.1371/journal.pone.0259731
Modelling the concentration of anti-SARS-CoV-2 immunoglobulin G in intravenous immunoglobulin product batches
Abstract
Background: Plasma-derived intravenous immunoglobulin (IVIg) products contain a dynamic spectrum of immunoglobulin (Ig) G reactivities reflective of the donor population from which they are derived. We sought to model the concentration of anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) IgG which could be expected in future plasma pool and final-product batches of CSL Behring's immunoglobulin product Privigen.
Study design and methods: Data was extracted from accessible databases, including the incidence of coronavirus disease 2019 and SARS-CoV-2 vaccination status, antibody titre in convalescent and vaccinated groups and antibody half-life. Together, these parameters were used to create an integrated mathematical model that could be used to predict anti-SARS-CoV-2 antibody levels in future IVIg preparations.
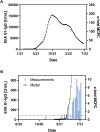
Results: We predict that anti-SARS-CoV-2 IgG concentration will peak in batches produced in mid-October 2021, containing levels in the vicinity of 190-fold that of the mean convalescent (unvaccinated) plasma concentration. An elevated concentration (approximately 35-fold convalescent plasma) is anticipated to be retained in batches produced well into 2022. Measurement of several Privigen batches using the Phadia™ EliA™ SARS-CoV-2-Sp1 IgG binding assay confirmed the early phase of this model.
Conclusion: The work presented in this paper may have important implications for physicians and patients who use Privigen for indicated diseases.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: SS, TB, PV, WM, DG, TH, SM, SP, NR and PS are employees of CSL Behring. JSP, DF and LS are employees of Thermo Fisher Scientific ImmunoDiagnostics Phadia, GmbH.
Figures
Similar articles
-
Anti-SARS-CoV-2 Antibodies Within IVIg Preparations: Cross-Reactivities With Seasonal Coronaviruses, Natural Autoimmunity, and Therapeutic Implications.Front Immunol. 2021 Feb 17;12:627285. doi: 10.3389/fimmu.2021.627285. eCollection 2021. Front Immunol. 2021. PMID: 33679770 Free PMC article.
-
Characterization of antibody responses to SARS-CoV-2 in convalescent COVID-19 patients.J Med Virol. 2021 Apr;93(4):2227-2233. doi: 10.1002/jmv.26646. Epub 2020 Nov 10. J Med Virol. 2021. PMID: 33135795
-
SARS-CoV-2 spike antibody concentration in gamma globulin products from high-prevalence COVID-19 countries are transmitted to X-linked agammaglobulinemia patients.Front Immunol. 2023 Mar 29;14:1156823. doi: 10.3389/fimmu.2023.1156823. eCollection 2023. Front Immunol. 2023. PMID: 37063907 Free PMC article.
-
Kinetics of SARS-CoV-2 Specific and Neutralizing Antibodies over Seven Months after Symptom Onset in COVID-19 Patients.Microbiol Spectr. 2021 Oct 31;9(2):e0059021. doi: 10.1128/Spectrum.00590-21. Epub 2021 Sep 22. Microbiol Spectr. 2021. PMID: 34550000 Free PMC article.
-
SARS-CoV-2 Antibody Longitudinal Profile of Immune Globulin Preparations.Mil Med. 2023 Jul 22;188(7-8):1615-1619. doi: 10.1093/milmed/usac192. Mil Med. 2023. PMID: 35769049 Free PMC article.
Cited by
-
Total escape of SARS-CoV-2 from dual monoclonal antibody therapy in an immunocompromised patient.Nat Commun. 2023 Apr 10;14(1):1999. doi: 10.1038/s41467-023-37591-w. Nat Commun. 2023. PMID: 37037847 Free PMC article.
-
An Overview of the Strategies to Boost SARS-CoV-2-Specific Immunity in People with Inborn Errors of Immunity.Vaccines (Basel). 2024 Jun 18;12(6):675. doi: 10.3390/vaccines12060675. Vaccines (Basel). 2024. PMID: 38932404 Free PMC article. Review.
-
The arrival of SARS-CoV-2-neutralizing antibodies in a currently available commercial immunoglobulin.J Allergy Clin Immunol. 2022 Jun;149(6):1958-1959. doi: 10.1016/j.jaci.2022.03.026. Epub 2022 Apr 22. J Allergy Clin Immunol. 2022. PMID: 35465974 Free PMC article. No abstract available.
-
A Case of Autoimmune Small Fiber Neuropathy as Possible Post COVID Sequelae.Int J Environ Res Public Health. 2023 Mar 10;20(6):4918. doi: 10.3390/ijerph20064918. Int J Environ Res Public Health. 2023. PMID: 36981826 Free PMC article.
References
-
- Simon TL, FitzGerald P, Kuhne M, Farrar C, Knowles J, Bycholski K, et al.. Longitudinal changes in measles antibody titers in plasma donors and minimum antibody levels of immunoglobulin products for treatment of primary immunodeficiency. Transfusion. 2018;58 Suppl 3:3065–71. doi: 10.1111/trf.15014 - DOI - PubMed
-
- Centers for Disease Control and Prevention (CDC). CDC COVID Data Tracker 2021 https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed 22 June, 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

