Protective Effects of Nootkatone on Renal Inflammation, Apoptosis, and Fibrosis in a Unilateral Ureteral Obstructive Mouse Model
- PMID: 34836176
- PMCID: PMC8621682
- DOI: 10.3390/nu13113921
Protective Effects of Nootkatone on Renal Inflammation, Apoptosis, and Fibrosis in a Unilateral Ureteral Obstructive Mouse Model
Abstract
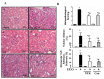
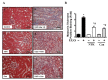
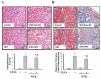
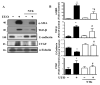
Nootkatone is one of the major active ingredients of Alpiniae oxyphyllae, which has been used as both food and medicinal plants for the treatment of diarrhea, ulceration, and enuresis. In this study, we aimed to investigate whether nootkatone treatment ameliorated the progression of chronic kidney diseases (CKD) and clarified its underlying mechanisms in an obstructive nephropathy (unilateral ureteral obstructive; UUO) mouse model. Our results revealed that nootkatone treatment preventively decreased the pathological changes and significantly mitigated the collagen deposition as well as the protein expression of fibrotic markers. Nootkatone could also alleviate oxidative stress-induced injury, inflammatory cell infiltration, and renal cell apoptotic death in the kidneys of UUO mice. These results demonstrated for the first time that nootkatone protected against the progression of CKD in a UUO mouse model. It may serve as a potential therapeutic candidate for CKD intervention.
Keywords: apoptosis; chronic kidney disease; inflammation; nootkatone; renal fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Withaferin A protects against endoplasmic reticulum stress-associated apoptosis, inflammation, and fibrosis in the kidney of a mouse model of unilateral ureteral obstruction.Phytomedicine. 2020 Dec;79:153352. doi: 10.1016/j.phymed.2020.153352. Epub 2020 Sep 21. Phytomedicine. 2020. PMID: 33007732
-
Wogonin ameliorates ER stress-associated inflammatory response, apoptotic death and renal fibrosis in a unilateral ureteral obstruction mouse model.Eur J Pharmacol. 2024 Aug 15;977:176676. doi: 10.1016/j.ejphar.2024.176676. Epub 2024 May 28. Eur J Pharmacol. 2024. PMID: 38815787
-
Renal Fibrosis, Immune Cell Infiltration and Changes of TRPC Channel Expression after Unilateral Ureteral Obstruction in Trpc6-/- Mice.Cell Physiol Biochem. 2019;52(6):1484-1502. doi: 10.33594/000000103. Cell Physiol Biochem. 2019. PMID: 31099508
-
Unilateral Ureteral Obstruction as a Model to Investigate Fibrosis-Attenuating Treatments.Biomolecules. 2019 Apr 8;9(4):141. doi: 10.3390/biom9040141. Biomolecules. 2019. PMID: 30965656 Free PMC article. Review.
-
Mitochondrial dysfunction and endoplasmic reticulum stress in the promotion of fibrosis in obstructive nephropathy induced by unilateral ureteral obstruction.Biofactors. 2020 Sep;46(5):716-733. doi: 10.1002/biof.1673. Epub 2020 Sep 9. Biofactors. 2020. PMID: 32905648 Review.
Cited by
-
Nootkatone Counteracts Melamine-Mediated Nephrotoxicity via Modulation of Intermediate Filament Proteins, Oxidative, Inflammatory, and Apoptotic Events.Drug Des Devel Ther. 2024 Jul 16;18:2989-3004. doi: 10.2147/DDDT.S466286. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39050805 Free PMC article.
-
Potential Therapeutic Strategies for Renal Fibrosis: Cordyceps and Related Products.Front Pharmacol. 2022 Jul 8;13:932172. doi: 10.3389/fphar.2022.932172. eCollection 2022. Front Pharmacol. 2022. PMID: 35873549 Free PMC article. Review.
-
Physicochemical Characterization and Biological Properties of Polysaccharides from Alpiniae oxyphyllae Fructus.Polymers (Basel). 2024 Jun 14;16(12):1705. doi: 10.3390/polym16121705. Polymers (Basel). 2024. PMID: 38932054 Free PMC article.
-
Nootkatone Supplementation Attenuates Carbon Tetrachloride Exposure-Induced Nephrotoxicity in Mice.Antioxidants (Basel). 2023 Feb 3;12(2):370. doi: 10.3390/antiox12020370. Antioxidants (Basel). 2023. PMID: 36829928 Free PMC article.
-
Nootkatone Supplementation Ameliorates Carbon Tetrachloride-Induced Acute Liver Injury via the Inhibition of Oxidative Stress, NF-κB Pathways, and the Activation of Nrf2/HO-1 Pathway.Antioxidants (Basel). 2023 Jan 13;12(1):194. doi: 10.3390/antiox12010194. Antioxidants (Basel). 2023. PMID: 36671056 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

