Cellulosic Polymers for Enhancing Drug Bioavailability in Ocular Drug Delivery Systems
- PMID: 34832983
- PMCID: PMC8621906
- DOI: 10.3390/ph14111201
Cellulosic Polymers for Enhancing Drug Bioavailability in Ocular Drug Delivery Systems
Abstract
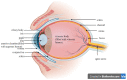
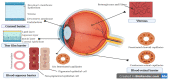
One of the major impediments to drug development is low aqueous solubility and thus poor bioavailability, which leads to insufficient clinical utility. Around 70-80% of drugs in the discovery pipeline are suffering from poor aqueous solubility and poor bioavailability, which is a major challenge when one has to develop an ocular drug delivery system. The outer lipid layer, pre-corneal, dynamic, and static ocular barriers limit drug availability to the targeted ocular tissues. Biopharmaceutical Classification System (BCS) class II drugs with adequate permeability and limited or no aqueous solubility have been extensively studied for various polymer-based solubility enhancement approaches. The hydrophilic nature of cellulosic polymers and their tunable properties make them the polymers of choice in various solubility-enhancement techniques. This review focuses on various cellulose derivatives, specifically, their role, current status and novel modified cellulosic polymers for enhancing the bioavailability of BCS class II drugs in ocular drug delivery systems.
Keywords: bioavailability; carboxymethyl cellulose; ethylcellulose; hydroxyethyl cellulose; hydroxypropyl methylcellulose; methylcellulose; ocular drug delivery system; solubility; sustained release.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Solid Lipid Nanoparticles: A Potential Option for Enhancing Oral Bioavailability of Highly Soluble and Poorly Permeable (BCS Class III) Drugs.Curr Drug Deliv. 2023;20(3):223-236. doi: 10.2174/1567201819666220418100410. Curr Drug Deliv. 2023. PMID: 35443896
-
Biodegradable ocular inserts for sustained delivery of brimonidine tartarate: preparation and in vitro/in vivo evaluation.AAPS PharmSciTech. 2011 Dec;12(4):1335-47. doi: 10.1208/s12249-011-9701-3. Epub 2011 Oct 7. AAPS PharmSciTech. 2011. PMID: 21979886 Free PMC article.
-
BCS class IV drugs: Highly notorious candidates for formulation development.J Control Release. 2017 Feb 28;248:71-95. doi: 10.1016/j.jconrel.2017.01.014. Epub 2017 Jan 11. J Control Release. 2017. PMID: 28088572 Review.
-
Impact of Physicochemical Properties of Cellulosic Polymers on Supersaturation Maintenance in Aqueous Drug Solutions.AAPS PharmSciTech. 2018 May;19(4):1860-1868. doi: 10.1208/s12249-018-0999-y. Epub 2018 Apr 10. AAPS PharmSciTech. 2018. PMID: 29637498
-
HPMC- A Marvel Polymer for Pharmaceutical Industry-Patent Review.Recent Adv Drug Deliv Formul. 2021;15(1):46-58. doi: 10.2174/1872211314666210604120619. Recent Adv Drug Deliv Formul. 2021. PMID: 34086557 Review.
Cited by
-
Overview of processed excipients in ocular drug delivery: Opportunities so far and bottlenecks.Heliyon. 2023 Dec 23;10(1):e23810. doi: 10.1016/j.heliyon.2023.e23810. eCollection 2024 Jan 15. Heliyon. 2023. PMID: 38226207 Free PMC article. Review.
-
Balsam Poplar Buds: Extraction of Potential Phenolic Compounds with Polyethylene Glycol Aqueous Solution, Thermal Sterilization of Extracts and Challenges to Their Application in Topical Ocular Formulations.Antioxidants (Basel). 2022 Sep 8;11(9):1771. doi: 10.3390/antiox11091771. Antioxidants (Basel). 2022. PMID: 36139845 Free PMC article.
-
Breaking Barriers in Eye Treatment: Polymeric Nano-Based Drug-Delivery System for Anterior Segment Diseases and Glaucoma.Polymers (Basel). 2023 Mar 9;15(6):1373. doi: 10.3390/polym15061373. Polymers (Basel). 2023. PMID: 36987154 Free PMC article. Review.
-
Chitosan nanoparticles laden contact lenses for enzyme-triggered controlled delivery of timolol maleate: A promising strategy for managing glaucoma.Drug Deliv Transl Res. 2024 Nov;14(11):3212-3224. doi: 10.1007/s13346-024-01543-8. Epub 2024 Feb 26. Drug Deliv Transl Res. 2024. PMID: 38407770
-
Functionalization and Antibacterial Applications of Cellulose-Based Composite Hydrogels.Polymers (Basel). 2022 Feb 16;14(4):769. doi: 10.3390/polym14040769. Polymers (Basel). 2022. PMID: 35215680 Free PMC article. Review.
References
-
- Suri R., Beg S., Kohli K. Target strategies for drug delivery bypassing ocular barriers. J. Drug Deliv. Sci. Technol. 2020;55:101389. doi: 10.1016/j.jddst.2019.101389. - DOI
Publication types
LinkOut - more resources
Full Text Sources