Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy
- PMID: 34831435
- PMCID: PMC8625920
- DOI: 10.3390/cells10113213
Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy
Abstract
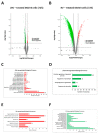
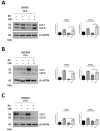
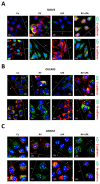
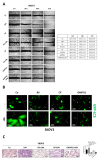
Background: Ovarian cancer progression and invasiveness are promoted by a range of soluble factors released by cancer cells and stromal cells within the tumor microenvironment. Our previous studies demonstrated that resveratrol (RV), a nutraceutical and caloric restriction mimetic with tumor-suppressive properties, counteracts cancer cell motility induced by stromal IL-6 by upregulating autophagy. Lysophosphatidic acid (LPA), a bioactive phospholipid that shows elevated levels in the tumor microenvironment and the ascites of ovarian cancers, stimulates the growth and tissue invasion of cancer cells. Whether LPA elicits these effects by inhibiting autophagy and through which pathway and whether RV can counteract the same remain obscure. Aims: To investigate the molecular pathways involved in LPA-induced ovarian cancer malignancy, particularly focusing on the role of autophagy, and the ability of RV to counteract LPA activity. Results: LPA stimulated while RV inhibited ovarian cancer cell migration. Transcriptomic and bioinformatic analyses showed an opposite regulation by LPA and RV of genes linked to epithelial-to-mesenchymal transition (EMT) and autophagy with involvement of the PI3K-AKT, JAK-STAT and Hedgehog (Hh) pathways. LPA upregulated the Hh and EMT members GLI1, BMI-1, SNAIL-1 and TWIST1 and inhibited autophagy, while RV did the opposite. Similar to the inhibitors of the Hh pathway, RV inhibited LPA-induced cancer cell migration and 3D growth of ovarian cancer cells. BMI-1 silencing prevented LPA-induced EMT, restored autophagy and hampered cell migration, resembling the effects of RV. TCGA data analyses indicated that patients with low expression of Hh/EMT-related genes together with active autophagy flux tended to have a better prognosis and this correlates with a more effective response to platinum therapy. In in vitro 3D spheroids, LPA upregulated BMI-1, downregulated autophagy and inhibited platinum toxicity while RV and Hh inhibitors restored autophagy and favored BAX-mediated cell death in response to platinum. Conclusions: By inhibiting the Hh pathway and restoration of autophagy, RV counteracts LPA-induced malignancy, supporting its inclusion in the therapy of ovarian cancer for limiting metastasis and chemoresistance.
Keywords: 3D spheroids; BMI-1; autophagy; cell migration; chemoresistance; epithelial to mesenchymal transition; overall survival; personalized cancer therapy; transcriptomic; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
NANOG regulates epithelial-mesenchymal transition and chemoresistance through activation of the STAT3 pathway in epithelial ovarian cancer.Tumour Biol. 2016 Jul;37(7):9671-80. doi: 10.1007/s13277-016-4848-x. Epub 2016 Jan 22. Tumour Biol. 2016. PMID: 26801672
-
NKX3-2 Induces Ovarian Cancer Cell Migration by HDAC6-Mediated Repositioning of Lysosomes and Inhibition of Autophagy.Cells. 2024 Nov 4;13(21):1816. doi: 10.3390/cells13211816. Cells. 2024. PMID: 39513923 Free PMC article.
-
HPIP promotes epithelial-mesenchymal transition and cisplatin resistance in ovarian cancer cells through PI3K/AKT pathway activation.Cell Oncol (Dordr). 2017 Apr;40(2):133-144. doi: 10.1007/s13402-016-0308-2. Epub 2016 Dec 30. Cell Oncol (Dordr). 2017. PMID: 28039608
-
Hedgehog Signaling Pathway and Autophagy in Cancer.Int J Mol Sci. 2018 Aug 3;19(8):2279. doi: 10.3390/ijms19082279. Int J Mol Sci. 2018. PMID: 30081498 Free PMC article. Review.
-
Snail transcription factors as key regulators of chemoresistance, stemness and metastasis of ovarian cancer cells.Biochim Biophys Acta Rev Cancer. 2023 Nov;1878(6):189003. doi: 10.1016/j.bbcan.2023.189003. Epub 2023 Oct 18. Biochim Biophys Acta Rev Cancer. 2023. PMID: 37863122 Review.
Cited by
-
Friend and foe: the regulation network of ascites components in ovarian cancer progression.J Cell Commun Signal. 2023 Sep;17(3):391-407. doi: 10.1007/s12079-022-00698-8. Epub 2022 Oct 13. J Cell Commun Signal. 2023. PMID: 36227507 Free PMC article. Review.
-
Focusing on the Role of Natural Products in Overcoming Cancer Drug Resistance: An Autophagy-Based Perspective.Biomolecules. 2022 Oct 26;12(11):1565. doi: 10.3390/biom12111565. Biomolecules. 2022. PMID: 36358919 Free PMC article. Review.
-
The prognostic activity of acylglycerol kinase immunohistochemical expression in colon adenocarcinoma patients.Prz Gastroenterol. 2023;18(4):430-436. doi: 10.5114/pg.2023.133477. Epub 2023 Dec 8. Prz Gastroenterol. 2023. PMID: 38572459 Free PMC article.
-
Nutriepigenomics in Environmental-Associated Oxidative Stress.Antioxidants (Basel). 2023 Mar 21;12(3):771. doi: 10.3390/antiox12030771. Antioxidants (Basel). 2023. PMID: 36979019 Free PMC article. Review.
-
Butyrate Inhibits Colorectal Cancer Cell Proliferation through Autophagy Degradation of β-Catenin Regardless of APC and β-Catenin Mutational Status.Biomedicines. 2022 May 13;10(5):1131. doi: 10.3390/biomedicines10051131. Biomedicines. 2022. PMID: 35625868 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

