Endothelial Dysfunction through Oxidatively Generated Epigenetic Mark in Respiratory Viral Infections
- PMID: 34831290
- PMCID: PMC8623825
- DOI: 10.3390/cells10113067
Endothelial Dysfunction through Oxidatively Generated Epigenetic Mark in Respiratory Viral Infections
Abstract
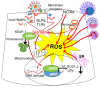
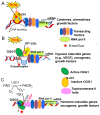
The bronchial vascular endothelial network plays important roles in pulmonary pathology during respiratory viral infections, including respiratory syncytial virus (RSV), influenza A(H1N1) and importantly SARS-Cov-2. All of these infections can be severe and even lethal in patients with underlying risk factors.A major obstacle in disease prevention is the lack of appropriate efficacious vaccine(s) due to continuous changes in the encoding capacity of the viral genome, exuberant responsiveness of the host immune system and lack of effective antiviral drugs. Current management of these severe respiratory viral infections is limited to supportive clinical care. The primary cause of morbidity and mortality is respiratory failure, partially due to endothelial pulmonary complications, including edema. The latter is induced by the loss of alveolar epithelium integrity and by pathological changes in the endothelial vascular network that regulates blood flow, blood fluidity, exchange of fluids, electrolytes, various macromolecules and responses to signals triggered by oxygenation, and controls trafficking of leukocyte immune cells. This overview outlines the latest understanding of the implications of pulmonary vascular endothelium involvement in respiratory distress syndrome secondary to viral infections. In addition, the roles of infection-induced cytokines, growth factors, and epigenetic reprogramming in endothelial permeability, as well as emerging treatment options to decrease disease burden, are discussed.
Keywords: SARS-Cov-2; endothelial cells; gene expression; influenza H1N1; oxidative stress; pulmonary edema; respiratory distress syndrome; respiratory syncytial virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Human pulmonary microvascular endothelial cells support productive replication of highly pathogenic avian influenza viruses: possible involvement in the pathogenesis of human H5N1 virus infection.J Virol. 2012 Jan;86(2):667-78. doi: 10.1128/JVI.06348-11. Epub 2011 Nov 9. J Virol. 2012. PMID: 22072765 Free PMC article.
-
Influenza A(H1N1)pdm09 Virus but Not Respiratory Syncytial Virus Interferes with SARS-CoV-2 Replication during Sequential Infections in Human Nasal Epithelial Cells.Viruses. 2022 Feb 15;14(2):395. doi: 10.3390/v14020395. Viruses. 2022. PMID: 35215988 Free PMC article.
-
Postinfection A77-1726 treatment improves cardiopulmonary function in H1N1 influenza-infected mice.Am J Respir Cell Mol Biol. 2012 Oct;47(4):543-51. doi: 10.1165/rcmb.2012-0112OC. Epub 2012 Jun 7. Am J Respir Cell Mol Biol. 2012. PMID: 22679275 Free PMC article.
-
Pathophysiological Association of Endothelial Dysfunction with Fatal Outcome in COVID-19.Int J Mol Sci. 2021 May 12;22(10):5131. doi: 10.3390/ijms22105131. Int J Mol Sci. 2021. PMID: 34066226 Free PMC article. Review.
-
Endothelial Damage in Acute Respiratory Distress Syndrome.Int J Mol Sci. 2020 Nov 20;21(22):8793. doi: 10.3390/ijms21228793. Int J Mol Sci. 2020. PMID: 33233715 Free PMC article. Review.
Cited by
-
COVID-19 and Pulmonary Angiogenesis: The Possible Role of Hypoxia and Hyperinflammation in the Overexpression of Proteins Involved in Alveolar Vascular Dysfunction.Viruses. 2023 Mar 8;15(3):706. doi: 10.3390/v15030706. Viruses. 2023. PMID: 36992415 Free PMC article.
-
OGG1 as an Epigenetic Reader Affects NFκB: What This Means for Cancer.Cancers (Basel). 2023 Dec 28;16(1):148. doi: 10.3390/cancers16010148. Cancers (Basel). 2023. PMID: 38201575 Free PMC article. Review.
-
The DNA glycosylase NEIL2 is protective during SARS-CoV-2 infection.Nat Commun. 2023 Dec 9;14(1):8169. doi: 10.1038/s41467-023-43938-0. Nat Commun. 2023. PMID: 38071370 Free PMC article.
-
Vascular dysfunction in COVID-19 patients: update on SARS-CoV-2 infection of endothelial cells and the role of long non-coding RNAs.Clin Sci (Lond). 2022 Nov 11;136(21):1571-1590. doi: 10.1042/CS20220235. Clin Sci (Lond). 2022. PMID: 36367091 Free PMC article.
References
-
- Aird W.C. Phenotypic Heterogeneity of the Endothelium. Circ. Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

