Pulmonary Alveolar Stem Cell Senescence, Apoptosis, and Differentiation by p53-Dependent and -Independent Mechanisms in Telomerase-Deficient Mice
- PMID: 34831112
- PMCID: PMC8616483
- DOI: 10.3390/cells10112892
Pulmonary Alveolar Stem Cell Senescence, Apoptosis, and Differentiation by p53-Dependent and -Independent Mechanisms in Telomerase-Deficient Mice
Abstract
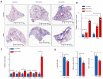
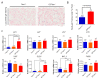
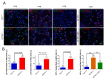
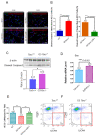
Pulmonary premature ageing and fibrogenesis as in idiopathic pulmonary fibrosis (IPF) occur with the DNA damage response in lungs deficient of telomerase. The molecular mechanism mediating pulmonary alveolar cell fates remains to be investigated. The present study shows that naturally occurring ageing is associated with the DNA damage response (DDR) and activation of the p53 signalling pathway. Telomerase deficiency induced by telomerase RNA component (TERC) knockout (KO) accelerates not only replicative senescence but also altered differentiation and apoptosis of the pulmonary alveolar stem cells (AEC2) in association with increased innate immune natural killer (NK) cells in TERC KO mice. TERC KO results in increased senescence-associated heterochromatin foci (SAHF) marker HP1γ, p21, p16, and apoptosis-associated cleaved caspase-3 in AEC2. However, additional deficiency of the tumour suppressor p53 in the Trp53-/- allele of the late generation of TERC KO mice attenuates the increased senescent and apoptotic markers significantly. Moreover, p53 deficiency has no significant effect on the increased gene expression of T1α (a marker of terminal differentiated AEC1) in AEC2 of the late generation of TERC KO mice. These findings demonstrate that, in natural ageing or premature ageing accelerated by telomere shortening, pulmonary senescence and IPF develop with alveolar stem cell p53-dependent premature replicative senescence, apoptosis, and p53-independent differentiation, resulting in pulmonary senescence-associated low-grade inflammation (SALI). Our studies indicate a natural ageing-associated molecular mechanism of telomerase deficiency-induced telomere DDR and SALI in pulmonary ageing and IPF.
Keywords: apoptosis; differentiation; lung alveolar type 2 cells; p53; senescence; telomerase RNA component; telomere shortening.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Molecular Mechanisms of Alveolar Epithelial Stem Cell Senescence and Senescence-Associated Differentiation Disorders in Pulmonary Fibrosis.Cells. 2022 Mar 3;11(5):877. doi: 10.3390/cells11050877. Cells. 2022. PMID: 35269498 Free PMC article. Review.
-
Pulmonary alveolar stem cells undergo senescence, apoptosis and differentiation by p53-dependent and -independent mechanisms in telomerase deficient mice.Clin Exp Pharmacol Physiol. 2021 May;48(5):651-659. doi: 10.1111/1440-1681.13472. Epub 2021 Feb 25. Clin Exp Pharmacol Physiol. 2021. Retraction in: Clin Exp Pharmacol Physiol. 2021 Sep;48(9):1301. doi: 10.1111/1440-1681.13559 PMID: 33634502 Retracted.
-
Serpine 1 induces alveolar type II cell senescence through activating p53-p21-Rb pathway in fibrotic lung disease.Aging Cell. 2017 Oct;16(5):1114-1124. doi: 10.1111/acel.12643. Epub 2017 Jul 19. Aging Cell. 2017. PMID: 28722352 Free PMC article.
-
Telomerase Deficiency Causes Alveolar Stem Cell Senescence-associated Low-grade Inflammation in Lungs.J Biol Chem. 2015 Dec 25;290(52):30813-29. doi: 10.1074/jbc.M115.681619. Epub 2015 Oct 30. J Biol Chem. 2015. PMID: 26518879 Free PMC article.
-
Control of Cellular Aging, Tissue Function, and Cancer by p53 Downstream of Telomeres.Cold Spring Harb Perspect Med. 2017 May 1;7(5):a026088. doi: 10.1101/cshperspect.a026088. Cold Spring Harb Perspect Med. 2017. PMID: 28289249 Free PMC article. Review.
Cited by
-
Gene expression profiling of RIP2-knockdown in HD11 macrophages - elucidation of potential pathways (gene network) when challenged with avian pathogenic E.coli (APEC).BMC Genomics. 2022 May 2;23(1):341. doi: 10.1186/s12864-022-08595-5. BMC Genomics. 2022. PMID: 35501708 Free PMC article.
-
Senescence of alveolar epithelial progenitor cells: a critical driver of lung fibrosis.Am J Physiol Cell Physiol. 2023 Aug 1;325(2):C483-C495. doi: 10.1152/ajpcell.00239.2023. Epub 2023 Jul 17. Am J Physiol Cell Physiol. 2023. PMID: 37458437 Free PMC article. Review.
-
Molecular Mechanisms of Alveolar Epithelial Stem Cell Senescence and Senescence-Associated Differentiation Disorders in Pulmonary Fibrosis.Cells. 2022 Mar 3;11(5):877. doi: 10.3390/cells11050877. Cells. 2022. PMID: 35269498 Free PMC article. Review.
-
The landscape of aging.Sci China Life Sci. 2022 Dec;65(12):2354-2454. doi: 10.1007/s11427-022-2161-3. Epub 2022 Sep 2. Sci China Life Sci. 2022. PMID: 36066811 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

