Plasma Levels and Diagnostic Utility of VEGF in a Three-Year Follow-Up of Patients with Breast Cancer
- PMID: 34830735
- PMCID: PMC8624052
- DOI: 10.3390/jcm10225452
Plasma Levels and Diagnostic Utility of VEGF in a Three-Year Follow-Up of Patients with Breast Cancer
Abstract
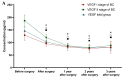
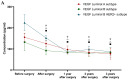
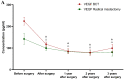
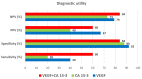
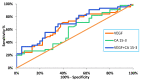
Breast cancer is the most common malignancy in women globally. The increasing worldwide incidence of this type of cancer illustrates the challenge it represents for healthcare providers. Therefore, new tumor markers are constantly being sought. The aim of this study was to assess plasma concentrations and the diagnostic power of VEGF in 100 patients with early-stage breast cancer, both before and after surgical treatment and during a three-year follow-up. The control groups included 50 subjects with benign breast tumors (fibroadenoma) and 50 healthy women. The VEGF concentration was determined using enzyme-linked immunosorbent assay (ELISA) and the CA 15-3 concentration was determined by chemiluminescent microparticle immunoassay (CMIA). We observed significantly higher preoperative plasma concentrations of VEGF and CA 15-3 in patients with breast cancer. VEGF, similar to CA 15-3, demonstrated high diagnostic utility in the assessment of the long-term efficacy of surgical removal of the tumor. Determinations of VEGF had the highest diagnostic usefulness in the detection of breast cancer recurrence (SE 40%, SP 92%, PPV 67%, NPV 79%). Additionally, the highest values of SE, NPV and AUC were observed during the combined analysis with CA 15-3 (60%; 84%; 0.7074, respectively). Our study suggests a promising diagnostic utility of VEGF in the early stages of breast cancer and in the evaluation of the efficacy of the surgical treatment of breast cancer as well as the detection of breast cancer recurrence, particularly in a combined analysis with CA 15-3 as a new diagnostic panel.
Keywords: CA 15-3; VEGF; breast cancer; tumor marker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Plasma levels and diagnostic utility of VEGF, MMP-2 and TIMP-2 in the diagnostics of breast cancer patients.Biomarkers. 2017 Mar;22(2):157-164. doi: 10.1080/1354750X.2016.1252955. Epub 2016 Nov 9. Biomarkers. 2017. PMID: 27775427
-
Plasma levels and diagnostic utility of VEGF, MMP-9, and TIMP-1 in the diagnosis of patients with breast cancer.Onco Targets Ther. 2016 Feb 24;9:911-9. doi: 10.2147/OTT.S99959. eCollection 2016. Onco Targets Ther. 2016. PMID: 26966379 Free PMC article.
-
Plasma levels of VEGF-A, VEGF B, and VEGFR-1 and applicability of these parameters as tumor markers in diagnosis of breast cancer.Acta Biochim Pol. 2018 Dec 13;65(4):621-628. doi: 10.18388/abp.2018_2713. Acta Biochim Pol. 2018. PMID: 30543720
-
Human Plasma Levels of VEGF-A, VEGF-C, VEGF-D, their Soluble Receptor - VEGFR-2 and Applicability of these Parameters as Tumor Markers in the Diagnostics of Breast Cancer.Pathol Oncol Res. 2019 Oct;25(4):1477-1486. doi: 10.1007/s12253-018-0527-0. Epub 2018 Nov 1. Pathol Oncol Res. 2019. PMID: 30387014 Free PMC article.
-
Plasma Levels and Diagnostic Utility of M-CSF, MMP-2 and its Inhibitor TIMP-2 in the Diagnostics of Breast Cancer Patients.Clin Lab. 2016 Sep 1;62(9):1661-1669. doi: 10.7754/Clin.Lab.2016.160118. Clin Lab. 2016. PMID: 28164586
Cited by
-
Detection of IL-1β, VEGF and IL-4 with their novel genetic variations in breast cancer patients.Saudi J Biol Sci. 2023 Feb;30(2):103544. doi: 10.1016/j.sjbs.2022.103544. Epub 2022 Dec 23. Saudi J Biol Sci. 2023. PMID: 36619680 Free PMC article.
-
Plasma Levels of Metalloproteinase 3 (MMP-3) and Metalloproteinase 7 (MMP-7) as New Candidates for Tumor Biomarkers in Diagnostic of Breast Cancer Patients.J Clin Med. 2023 Mar 30;12(7):2618. doi: 10.3390/jcm12072618. J Clin Med. 2023. PMID: 37048701 Free PMC article.
-
Plasma Levels of CXC Motif Chemokine 1 (CXCL1) and Chemokine 8 (CXCL8) as Diagnostic Biomarkers in Luminal A and B Breast Cancer.J Clin Med. 2022 Nov 12;11(22):6694. doi: 10.3390/jcm11226694. J Clin Med. 2022. PMID: 36431173 Free PMC article.
-
Utility of Matrix Metalloproteinases in the Diagnosis, Monitoring and Prognosis of Ovarian Cancer Patients.Cancer Manag Res. 2022 Nov 30;14:3359-3382. doi: 10.2147/CMAR.S385658. eCollection 2022. Cancer Manag Res. 2022. PMID: 36474934 Free PMC article. Review.
-
Carbon Nanotube and Its Derived Nanomaterials Based High Performance Biosensing Platform.Biosensors (Basel). 2022 Sep 6;12(9):731. doi: 10.3390/bios12090731. Biosensors (Basel). 2022. PMID: 36140116 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Research Materials

