Presynaptic Release-Regulating Alpha2 Autoreceptors: Potential Molecular Target for Ellagic Acid Nutraceutical Properties
- PMID: 34829630
- PMCID: PMC8614955
- DOI: 10.3390/antiox10111759
Presynaptic Release-Regulating Alpha2 Autoreceptors: Potential Molecular Target for Ellagic Acid Nutraceutical Properties
Abstract
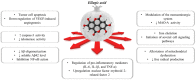
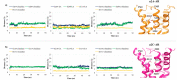
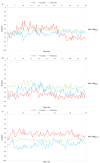
Polyphenol ellagic acid (EA) possesses antioxidant, anti-inflammatory, anti-carcinogenic, anti-diabetic and cardio protection activities, making it an interesting multi-targeting profile. EA also controls the central nervous system (CNS), since it was proven to reduce the immobility time of mice in both the forced swimming and the tail-suspension tests, with an efficiency comparable to that of classic antidepressants. Interestingly, the anti-depressant-like effect was almost nulled by the concomitant administration of selective antagonists of the noradrenergic receptors, suggesting the involvement of these cellular targets in the central effects elicited by EA and its derivatives. By in silico and in vitro studies, we discuss how EA engages with human α2A-ARs and α2C-AR catalytic pockets, comparing EA behaviour with that of known agonists and antagonists. Structurally, the hydrophobic residues surrounding the α2A-AR pocket confer specificity on the intermolecular interactions and hence lead to favourable binding of EA in the α2A-AR, with respect to α2C-AR. Moreover, EA seems to better accommodate within α2A-ARs into the TM5 area, close to S200 and S204, which play a crucial role for activation of aminergic GPCRs such as the α2-AR, highlighting its promising role as a partial agonist. Consistently, EA mimics clonidine in inhibiting noradrenaline exocytosis from hippocampal nerve endings in a yohimbine-sensitive fashion that confirms the engagement of naïve α2-ARs in the EA-mediated effect.
Keywords: antidepressant activity; antioxidant; ellagic acid; food chemistry; molecular dynamic simulations; molecular modelling; natural compounds; pomegranate tannins; α2-ARs; α2-adrenoreceptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
The anticonvulsant and proconvulsant effects of alpha2-adrenoreceptor agonists are mediated by distinct populations of alpha2A-adrenoreceptors.Neuroscience. 2004;126(3):795-803. doi: 10.1016/j.neuroscience.2004.04.030. Neuroscience. 2004. PMID: 15183527
-
A study of presynaptic alpha2-autoreceptors in alpha2A/D-, alpha2B- and alpha2C-adrenoceptor-deficient mice.Naunyn Schmiedebergs Arch Pharmacol. 2001 Aug;364(2):117-30. doi: 10.1007/s002100100423. Naunyn Schmiedebergs Arch Pharmacol. 2001. PMID: 11534851
-
Abnormal regulation of the sympathetic nervous system in alpha2A-adrenergic receptor knockout mice.Mol Pharmacol. 1999 Jul;56(1):154-61. doi: 10.1124/mol.56.1.154. Mol Pharmacol. 1999. PMID: 10385696
-
Therapeutic Potential of Selectively Targeting the α2C-Adrenoceptor in Cognition, Depression, and Schizophrenia-New Developments and Future Perspective.Front Psychiatry. 2017 Aug 14;8:144. doi: 10.3389/fpsyt.2017.00144. eCollection 2017. Front Psychiatry. 2017. PMID: 28855875 Free PMC article. Review.
-
Ellagic acid a multi-target bioactive compound for drug discovery in CNS? A narrative review.Eur J Med Chem. 2019 Dec 1;183:111724. doi: 10.1016/j.ejmech.2019.111724. Epub 2019 Sep 20. Eur J Med Chem. 2019. PMID: 31563012 Review.
Cited by
-
The Effects of Ellagic Acid on Experimental Corrosive Esophageal Burn Injury.Curr Issues Mol Biol. 2024 Feb 16;46(2):1579-1592. doi: 10.3390/cimb46020102. Curr Issues Mol Biol. 2024. PMID: 38392220 Free PMC article.
-
Ellagic Acid Prevents α-Synuclein Spread and Mitigates Toxicity by Enhancing Autophagic Flux in an Animal Model of Parkinson's Disease.Nutrients. 2023 Dec 26;16(1):85. doi: 10.3390/nu16010085. Nutrients. 2023. PMID: 38201915 Free PMC article.
-
Guanfacine Normalizes the Overexpression of Presynaptic α-2A Adrenoceptor Signaling and Ameliorates Neuropathic Pain in a Chronic Animal Model of Type 1 Diabetes.Pharmaceutics. 2022 Oct 10;14(10):2146. doi: 10.3390/pharmaceutics14102146. Pharmaceutics. 2022. PMID: 36297581 Free PMC article.
-
In Silico and In Vitro Study of Antioxidant Potential of Urolithins.Antioxidants (Basel). 2023 Mar 11;12(3):697. doi: 10.3390/antiox12030697. Antioxidants (Basel). 2023. PMID: 36978945 Free PMC article.
-
A Drug Discovery Approach to a Reveal Novel Antioxidant Natural Source: The Case of Chestnut Burr Biomass.Int J Mol Sci. 2024 Feb 21;25(5):2517. doi: 10.3390/ijms25052517. Int J Mol Sci. 2024. PMID: 38473765 Free PMC article.
References
-
- Turrini F., Malaspina P., Giordani P., Catena S., Zunin P., Boggia R. Traditional decoction and PUAE aqueous extracts of pomegranate peels as potential anti-tyrosinase ingredients. Appl. Sci. 2020;10:2795. doi: 10.3390/app10082795. - DOI
-
- Global Pomegranate Market Industry Trends, Sales, Supply, Demand, Analysis & Forecast. 2017. [(accessed on 17 November 2020)]. Available online: https://www.researchmoz.us/global-pomegranate-market-2017-industry-trend....
LinkOut - more resources
Full Text Sources
Research Materials

