Mechanism, Prevention, and Treatment of Radiation-Induced Salivary Gland Injury Related to Oxidative Stress
- PMID: 34829539
- PMCID: PMC8614677
- DOI: 10.3390/antiox10111666
Mechanism, Prevention, and Treatment of Radiation-Induced Salivary Gland Injury Related to Oxidative Stress
Abstract
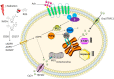
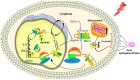
Radiation therapy is a common treatment for head and neck cancers. However, because of the presence of nerve structures (brain stem, spinal cord, and brachial plexus), salivary glands (SGs), mucous membranes, and swallowing muscles in the head and neck regions, radiotherapy inevitably causes damage to these normal tissues. Among them, SG injury is a serious adverse event, and its clinical manifestations include changes in taste, difficulty chewing and swallowing, oral infections, and dental caries. These clinical symptoms seriously reduce a patient's quality of life. Therefore, it is important to clarify the mechanism of SG injury caused by radiotherapy. Although the mechanism of radiation-induced SG injury has not yet been determined, recent studies have shown that the mechanisms of calcium signaling, microvascular injury, cellular senescence, and apoptosis are closely related to oxidative stress. In this article, we review the mechanism by which radiotherapy causes oxidative stress and damages the SGs. In addition, we discuss effective methods to prevent and treat radiation-induced SG damage.
Keywords: head and neck cancer; injury; oxidative stress; radiotherapy; salivary glands.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Mechanisms and protective measures for radiation-induced brachial plexus nerve injury.Brain Res Bull. 2024 May;210:110924. doi: 10.1016/j.brainresbull.2024.110924. Epub 2024 Mar 7. Brain Res Bull. 2024. PMID: 38460911 Review.
-
Dietary nitrate supplementation prevents radiotherapy-induced xerostomia.Elife. 2021 Sep 28;10:e70710. doi: 10.7554/eLife.70710. Elife. 2021. PMID: 34581269 Free PMC article.
-
A novel cell-based transplantation method using a Rho kinase inhibitor and a specific catheter device for the treatment of salivary gland damage after head and neck radiotherapy.Biochem Biophys Rep. 2022 Nov 12;32:101385. doi: 10.1016/j.bbrep.2022.101385. eCollection 2022 Dec. Biochem Biophys Rep. 2022. PMID: 36386443 Free PMC article.
-
Protective efficacy of intravenous transplantation of adipose-derived stem cells for the prevention of radiation-induced salivary gland damage.Arch Oral Biol. 2015 Oct;60(10):1488-96. doi: 10.1016/j.archoralbio.2015.07.016. Epub 2015 Jul 29. Arch Oral Biol. 2015. PMID: 26263537
-
Radiation-induced xerostomia in patients with head and neck cancer: pathogenesis, impact on quality of life, and management.Head Neck. 2004 Sep;26(9):796-807. doi: 10.1002/hed.20045. Head Neck. 2004. PMID: 15350026 Review.
Cited by
-
Salidroside Ameliorates Radiation Damage by Reducing Mitochondrial Oxidative Stress in the Submandibular Gland.Antioxidants (Basel). 2022 Jul 21;11(7):1414. doi: 10.3390/antiox11071414. Antioxidants (Basel). 2022. PMID: 35883904 Free PMC article.
-
Intragland Expression of the Shh Gene Alleviates Irradiation-Induced Salivary Gland Injury through Microvessel Protection and the Regulation of Oxidative Stress.Antioxidants (Basel). 2024 Jul 26;13(8):904. doi: 10.3390/antiox13080904. Antioxidants (Basel). 2024. PMID: 39199151 Free PMC article.
-
Cooperation effects of radiation and ferroptosis on tumor suppression and radiation injury.Front Cell Dev Biol. 2022 Sep 13;10:951116. doi: 10.3389/fcell.2022.951116. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36176274 Free PMC article. Review.
-
Tetrahedral framework nucleic acids alleviate irradiation-induced salivary gland damage.Cell Prolif. 2023 Apr;56(4):e13381. doi: 10.1111/cpr.13381. Epub 2022 Dec 13. Cell Prolif. 2023. PMID: 36514865 Free PMC article.
-
Mesenchymal stem cells in radiation-induced lung injury: From mechanisms to therapeutic potential.Front Cell Dev Biol. 2022 Dec 12;10:1100305. doi: 10.3389/fcell.2022.1100305. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36578783 Free PMC article. Review.
References
-
- Eisbruch A., Ten Haken R.K., Kim H.M., Marsh L.H., Ship J.A. Dose, volume, and function relationships in parotid salivary glands following conformal and intensi-ty-modulated irradiation of head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 1999;45:577–587. doi: 10.1016/S0360-3016(99)00247-3. - DOI - PubMed
-
- Tribius S., Haladyn S., Hanken H., Busch C.J., Krüll A., Petersen C., Bergelt C. Parotid sparing and quality of life in long-term survivors of locally advanced head and neck cancer after intensi-ty-modulated radiation therapy. Strahlenther. Onkol. 2021;197:219–230. doi: 10.1007/s00066-020-01737-2. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

