Inhibitors of Discoidin Domain Receptor (DDR) Kinases for Cancer and Inflammation
- PMID: 34827669
- PMCID: PMC8615839
- DOI: 10.3390/biom11111671
Inhibitors of Discoidin Domain Receptor (DDR) Kinases for Cancer and Inflammation
Abstract
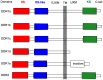
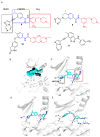
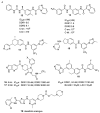
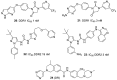
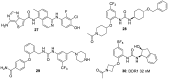
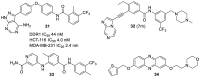
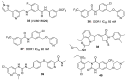
The discoidin domain receptor tyrosine kinases DDR1 and DDR2 are distinguished from other kinase enzymes by their extracellular domains, which interact with collagen rather than with peptidic growth factors, before initiating signaling via tyrosine phosphorylation. They share significant sequence and structural homology with both the c-Kit and Bcr-Abl kinases, and so many inhibitors of those kinases are also effective. Nevertheless, there has been an extensive research effort to develop potent and specific DDR inhibitors. A key interaction for many of these compounds is H-bonding to Met-704 in a hydrophobic pocket of the DDR enzyme. The most widespread use of DDR inhibitors has been for cancer therapy, but they have also shown effectiveness in animal models of inflammatory conditions such as Alzheimer's and Parkinson's diseases, and in chronic renal failure and glomerulonephritis.
Keywords: DDR kinase inhibitors; selectivity; small molecules.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

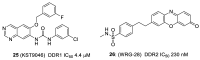
Similar articles
-
The Journey of DDR1 and DDR2 Kinase Inhibitors as Rising Stars in the Fight Against Cancer.Int J Mol Sci. 2021 Jun 18;22(12):6535. doi: 10.3390/ijms22126535. Int J Mol Sci. 2021. PMID: 34207360 Free PMC article. Review.
-
Discoidin domain receptors: Micro insights into macro assemblies.Biochim Biophys Acta Mol Cell Res. 2019 Nov;1866(11):118496. doi: 10.1016/j.bbamcr.2019.06.010. Epub 2019 Jun 21. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31229648 Free PMC article. Review.
-
Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib.Eur J Pharmacol. 2008 Dec 3;599(1-3):44-53. doi: 10.1016/j.ejphar.2008.10.014. Epub 2008 Oct 11. Eur J Pharmacol. 2008. PMID: 18938156
-
Using synthetic peptides and recombinant collagen to understand DDR-collagen interactions.Biochim Biophys Acta Mol Cell Res. 2019 Nov;1866(11):118458. doi: 10.1016/j.bbamcr.2019.03.005. Epub 2019 Mar 15. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30880148 Review.
-
Structural mechanisms determining inhibition of the collagen receptor DDR1 by selective and multi-targeted type II kinase inhibitors.J Mol Biol. 2014 Jun 26;426(13):2457-70. doi: 10.1016/j.jmb.2014.04.014. Epub 2014 Apr 23. J Mol Biol. 2014. PMID: 24768818 Free PMC article.
Cited by
-
E-Cadherin-Deficient Cells Are Sensitive to the Multikinase Inhibitor Dasatinib.Cancers (Basel). 2022 Mar 22;14(7):1609. doi: 10.3390/cancers14071609. Cancers (Basel). 2022. PMID: 35406381 Free PMC article.
-
SRC and TKS5 mediated podosome formation in fibroblasts promotes extracellular matrix invasion and pulmonary fibrosis.Nat Commun. 2023 Sep 21;14(1):5882. doi: 10.1038/s41467-023-41614-x. Nat Commun. 2023. PMID: 37735172 Free PMC article.
-
Genetic and pharmacological tools to study the role of discoidin domain receptors in kidney disease.Front Pharmacol. 2022 Sep 28;13:1001122. doi: 10.3389/fphar.2022.1001122. eCollection 2022. Front Pharmacol. 2022. PMID: 36249782 Free PMC article. Review.
-
Virtual screening for potential discoidin domain receptor 1 (DDR1) inhibitors based on structural assessment.Mol Divers. 2023 Oct;27(5):2297-2314. doi: 10.1007/s11030-022-10557-8. Epub 2022 Nov 2. Mol Divers. 2023. PMID: 36322341
References
-
- Carafoli F., Mayer M.C., Shiraishi K., Pecheva M.A., Chan L.Y., Nan R., Leitinger B., Hofmeister E.E. Structure of the Discoidin Domain Receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling. Structure. 2012;8:688–697. doi: 10.1016/j.str.2012.02.011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

