The Pseudo-Symmetric N-benzyl Hydroxyethylamine Core in a New Series of Heteroarylcarboxyamide HIV-1 Pr Inhibitors: Synthesis, Molecular Modeling and Biological Evaluation
- PMID: 34827582
- PMCID: PMC8615997
- DOI: 10.3390/biom11111584
The Pseudo-Symmetric N-benzyl Hydroxyethylamine Core in a New Series of Heteroarylcarboxyamide HIV-1 Pr Inhibitors: Synthesis, Molecular Modeling and Biological Evaluation
Abstract
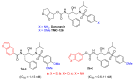
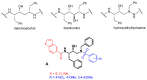
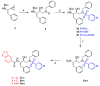
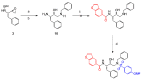
Here, we report the synthesis, enzyme inhibition and structure-activity relationship studies of a new potent class of HIV-1 protease inhibitors, which contain a pseudo-symmetric hydroxyethylamine core and heteroarylcarboxyamide moieties. The simple synthetic pathway furnished nine compounds in a few steps with high yields. The compounds were designed taking into account our previous results on other series of inhibitors with different substituents at P' and P'' and different ways of linking them to the inhibitor core. Potent inhibitory activity was obtained with nanomolar IC50 values measured with a standard fluorimetric test in 100 mM MES buffer, pH 5.5, containing 400 mM NaCl, 1 mM EDTA, 1 mM DTT and 1 mg/ml BSA. Compounds 9a-c, containing the indole ring in P1, exhibited an HIV-1 protease inhibitory activity more powerful than darunavir in the same assay. To obtain molecular insight into the binding properties of these compounds, docking analysis was performed, and their binding properties were also compared.
Keywords: HIV-protease inhibitors; biological screening; heteroaryl carboxyamides; modeling; pseudo-symmetric core; synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Synthesis and biological evaluation of novel amprenavir-based P1-substituted bi-aryl derivatives as ultra-potent HIV-1 protease inhibitors.Bioorg Med Chem Lett. 2012 Mar 1;22(5):1976-9. doi: 10.1016/j.bmcl.2012.01.037. Epub 2012 Jan 21. Bioorg Med Chem Lett. 2012. PMID: 22306123
-
A symmetric inhibitor binds HIV-1 protease asymmetrically.Biochemistry. 1993 Jan 26;32(3):937-47. doi: 10.1021/bi00054a027. Biochemistry. 1993. PMID: 8422397
-
Design, synthesis, and biological and structural evaluations of novel HIV-1 protease inhibitors to combat drug resistance.J Med Chem. 2012 Jul 26;55(14):6328-41. doi: 10.1021/jm300238h. Epub 2012 Jul 13. J Med Chem. 2012. PMID: 22708897 Free PMC article.
-
Synthesis and biological evaluation in vitro and in mammalian cells of new heteroaryl carboxyamides as HIV-protease inhibitors.Bioorg Med Chem. 2017 Sep 1;25(17):4715-4722. doi: 10.1016/j.bmc.2017.07.014. Epub 2017 Jul 11. Bioorg Med Chem. 2017. PMID: 28739156
-
Resilience to resistance of HIV-1 protease inhibitors: profile of darunavir.AIDS Rev. 2008 Jul-Sep;10(3):131-42. AIDS Rev. 2008. PMID: 18820715 Free PMC article. Review.
Cited by
-
Targeting Viral and Cellular Cysteine Proteases for Treatment of New Variants of SARS-CoV-2.Viruses. 2024 Feb 22;16(3):338. doi: 10.3390/v16030338. Viruses. 2024. PMID: 38543704 Free PMC article.
References
-
- Global Report: UNAIDS Report on the Global AIDS Epidemic 2021. [(accessed on 17 September 2021)]. Available online: https://www.unaids.org/sites/default/files/media_asset/2021-global-aids-...
-
- World Health Organization, Geneva, Switzerland. [(accessed on 17 September 2021)]. Available online: https://www.who.int/hiv/pub/arv/chapter4.pdf.
-
- Ghosh A.K., Chapsal B.D. Aspartic acid proteases as therapeutic targets. In: Ghosh A.K., editor. Methods and Principles in Medicinal Chemistry. Volume 45. Wiley-VCH; Weinheim, Germany: 2010. pp. 169–204.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

