Cell membrane-anchored anti-HIV single-chain antibodies and bifunctional inhibitors targeting the gp41 fusion protein: new strategies for HIV gene therapy
- PMID: 34821542
- PMCID: PMC8735881
- DOI: 10.1080/22221751.2021.2011616
Cell membrane-anchored anti-HIV single-chain antibodies and bifunctional inhibitors targeting the gp41 fusion protein: new strategies for HIV gene therapy
Abstract
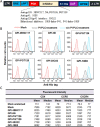
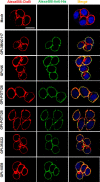
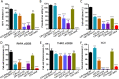
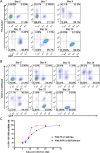
Emerging studies indicate that infusion of HIV-resistant cells could be an effective strategy to achieve a sterilizing or functional cure. We recently reported that glycosylphosphatidylinositol (GPI)-anchored nanobody or a fusion inhibitory peptide can render modified cells resistant to HIV-1 infection. In this study, we comprehensively characterized a panel of newly isolated HIV-1-neutralizing antibodies as GPI-anchored inhibitors. Fusion genes encoding the single-chain variable fragment (scFv) of 3BNC117, N6, PGT126, PGT128, 10E8, or 35O22 were constructed with a self-inactivating lentiviral vector, and they were efficiently expressed in the lipid raft sites of target cell membrane without affecting the expression of HIV-1 receptors (CD4, CCR5 and CXCR4). Significantly, transduced cells exhibited various degrees of resistance to cell-free HIV-1 infection and cell-associated HIV-1 transmission, as well as viral Env-mediated cell-cell fusion, with the cells modified by GPI-10E8 showing the most potent and broad anti-HIV activity. In mechanism, GPI-10E8 also interfered with the processing of viral Env in transduced cells and attenuated the infectivity of progeny viruses. By genetically linking 10E8 with a fusion inhibitor peptide, we subsequently designed a group of eight bifunctional constructs as cell membrane-based inhibitors, designated CMI01∼CMI08, which rendered cells completely resistant to HIV-1, HIV-2, and simian immunodeficiency virus (SIV). In human CD4+ T cells, GPI-10E8 and its bifunctional derivatives blocked both CCR5- and CXCR4-tropic HIV-1 isolates efficiently, and the modified cells displayed robust survival selection under HIV-1 infection. Therefore, our studies provide new strategies for generating HIV-resistant cells, which can be used alone or with other gene therapy approaches.
Keywords: HIV; broadly neutralizing antibodies (bNAbs); fusion inhibitory peptide; gene therapy; glycosylphosphatidylinositol (GPI).
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

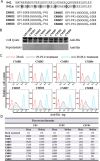
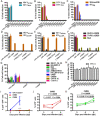
Similar articles
-
A Membrane-Anchored Short-Peptide Fusion Inhibitor Fully Protects Target Cells from Infections of Human Immunodeficiency Virus Type 1 (HIV-1), HIV-2, and Simian Immunodeficiency Virus.J Virol. 2019 Oct 29;93(22):e01177-19. doi: 10.1128/JVI.01177-19. Print 2019 Nov 15. J Virol. 2019. PMID: 31462566 Free PMC article.
-
Glycosylphosphatidylinositol-Anchored Anti-HIV scFv Efficiently Protects CD4 T Cells from HIV-1 Infection and Deletion in hu-PBL Mice.J Virol. 2017 Jan 18;91(3):e01389-16. doi: 10.1128/JVI.01389-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27881659 Free PMC article.
-
The Glycosylphosphatidylinositol-Anchored Variable Region of Llama Heavy Chain-Only Antibody JM4 Efficiently Blocks both Cell-Free and T Cell-T Cell Transmission of Human Immunodeficiency Virus Type 1.J Virol. 2016 Nov 14;90(23):10642-10659. doi: 10.1128/JVI.01559-16. Print 2016 Dec 1. J Virol. 2016. PMID: 27654286 Free PMC article.
-
Peptide and non-peptide HIV fusion inhibitors.Curr Pharm Des. 2002;8(8):563-80. doi: 10.2174/1381612024607180. Curr Pharm Des. 2002. PMID: 11945159 Review.
-
The molecular basis of HIV entry.Cell Microbiol. 2012 Aug;14(8):1183-92. doi: 10.1111/j.1462-5822.2012.01812.x. Epub 2012 Jun 5. Cell Microbiol. 2012. PMID: 22583677 Free PMC article. Review.
Cited by
-
Screening and affinity optimization of single domain antibody targeting the SARS-CoV-2 nucleocapsid protein.PeerJ. 2024 Aug 30;12:e17846. doi: 10.7717/peerj.17846. eCollection 2024. PeerJ. 2024. PMID: 39224822 Free PMC article.
-
Design of a Bispecific HIV Entry Inhibitor Targeting the Cell Receptor CD4 and Viral Fusion Protein Gp41.Front Cell Infect Microbiol. 2022 May 27;12:916487. doi: 10.3389/fcimb.2022.916487. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35711654 Free PMC article.
-
Clostridium innocuum, an emerging pathogen that induces lipid raft-mediated cytotoxicity.Virulence. 2023 Dec;14(1):2265048. doi: 10.1080/21505594.2023.2265048. Epub 2023 Oct 5. Virulence. 2023. PMID: 37798913 Free PMC article.
-
Combined Nano-Vector Mediated-Transfer to Suppress HIV-1 Infection with Targeted Antibodies in-vitro.Int J Nanomedicine. 2023 Aug 16;18:4635-4645. doi: 10.2147/IJN.S412915. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37605734 Free PMC article.
-
Functional Delineation of a Protein-Membrane Interaction Hotspot Site on the HIV-1 Neutralizing Antibody 10E8.Int J Mol Sci. 2022 Sep 15;23(18):10767. doi: 10.3390/ijms231810767. Int J Mol Sci. 2022. PMID: 36142694 Free PMC article.
References
-
- Barre-Sinoussi F, Ross AL, Delfraissy JF.. Past, present and future: 30 years of HIV research. Nat Rev Microbiol. 2013;11:877–883. - PubMed
-
- Collier DA, Monit C, Gupta RK.. The impact of HIV-1 drug escape on the global treatment landscape. Cell Host Microbe. 2019;26:48–60. - PubMed
-
- Hutter G, Nowak D, Mossner M, et al. . Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. - PubMed
-
- Peterson CW, Kiem HP.. Lessons from London and Berlin: designing A scalable gene therapy approach for HIV cure. Cell Stem Cell. 2019;24:685–687. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
