Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer
- PMID: 34819622
- PMCID: PMC9004227
- DOI: 10.1038/s41571-021-00579-w
Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer
Erratum in
-
Author Correction: Radiotherapy as a tool to elicit clinically actionable signalling pathways in cancer.Nat Rev Clin Oncol. 2022 Apr;19(4):281. doi: 10.1038/s41571-022-00611-7. Nat Rev Clin Oncol. 2022. PMID: 35181748 No abstract available.
Abstract
A variety of targeted anticancer agents have been successfully introduced into clinical practice, largely reflecting their ability to inhibit specific molecular alterations that are required for disease progression. However, not all malignant cells rely on such alterations to survive, proliferate, disseminate and/or evade anticancer immunity, implying that many tumours are intrinsically resistant to targeted therapies. Radiotherapy is well known for its ability to activate cytotoxic signalling pathways that ultimately promote the death of cancer cells, as well as numerous cytoprotective mechanisms that are elicited by cellular damage. Importantly, many cytoprotective mechanisms elicited by radiotherapy can be abrogated by targeted anticancer agents, suggesting that radiotherapy could be harnessed to enhance the clinical efficacy of these drugs. In this Review, we discuss preclinical and clinical data that introduce radiotherapy as a tool to elicit or amplify clinically actionable signalling pathways in patients with cancer.
© 2021. Springer Nature Limited.
Figures
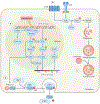
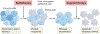
Similar articles
-
EGFR-targeted anti-cancer drugs in radiotherapy: preclinical evaluation of mechanisms.Radiother Oncol. 2007 Jun;83(3):238-48. doi: 10.1016/j.radonc.2007.04.006. Epub 2007 May 14. Radiother Oncol. 2007. PMID: 17502118 Review.
-
Immune-priming of the tumor microenvironment by radiotherapy: rationale for combination with immunotherapy to improve anticancer efficacy.Am J Clin Oncol. 2015 Feb;38(1):90-7. doi: 10.1097/COC.0b013e3182868ec8. Am J Clin Oncol. 2015. PMID: 25616204 Review.
-
Targeting apoptosis in cancer therapy.Nat Rev Clin Oncol. 2020 Jul;17(7):395-417. doi: 10.1038/s41571-020-0341-y. Epub 2020 Mar 23. Nat Rev Clin Oncol. 2020. PMID: 32203277 Free PMC article. Review.
-
Building immunity to cancer with radiation therapy.Cancer Lett. 2015 Nov 28;368(2):198-208. doi: 10.1016/j.canlet.2015.01.009. Epub 2015 Jan 12. Cancer Lett. 2015. PMID: 25592036 Review.
-
The role of autophagy in HER2-targeted therapy.Swiss Med Wkly. 2019 Oct 27;149:w20138. doi: 10.4414/smw.2019.20138. eCollection 2019 Oct 7. Swiss Med Wkly. 2019. PMID: 31656036 Review.
Cited by
-
Molecular mechanisms and therapeutic applications of huaier in breast cancer treatment.Front Pharmacol. 2024 Jan 12;14:1269096. doi: 10.3389/fphar.2023.1269096. eCollection 2023. Front Pharmacol. 2024. PMID: 38313074 Free PMC article. Review.
-
Targeting oncogene and non-oncogene addiction to inflame the tumour microenvironment.Nat Rev Drug Discov. 2022 Jun;21(6):440-462. doi: 10.1038/s41573-022-00415-5. Epub 2022 Mar 15. Nat Rev Drug Discov. 2022. PMID: 35292771 Review.
-
The role of p53 in anti-tumor immunity and response to immunotherapy.Front Mol Biosci. 2023 Aug 1;10:1148389. doi: 10.3389/fmolb.2023.1148389. eCollection 2023. Front Mol Biosci. 2023. PMID: 37602328 Free PMC article. Review.
-
Oncolytic mineralized bacteria as potent locally administered immunotherapeutics.Nat Biomed Eng. 2024 May;8(5):561-578. doi: 10.1038/s41551-024-01191-w. Epub 2024 Mar 21. Nat Biomed Eng. 2024. PMID: 38514774
-
RhoJ: an emerging biomarker and target in cancer research and treatment.Cancer Gene Ther. 2024 Oct;31(10):1454-1464. doi: 10.1038/s41417-024-00792-6. Epub 2024 Jun 10. Cancer Gene Ther. 2024. PMID: 38858534 Review.
References
-
- Bedard PL, Hyman DM, Davids MS & Siu LL Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 395, 1078–1088 (2020). - PubMed
-
- Boumahdi S & de Sauvage FJ The great escape: tumour cell plasticity in resistance to targeted therapy. Nat. Rev. Drug Discov 19, 39–56 (2020). - PubMed
-
- Sotorasib edges closer to approval. Cancer Discov. 11, OF2 (2021). - PubMed
-
- Doroshow DB et al. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol 18, 345–362 (2021). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

