ELAPOR1 is a secretory granule maturation-promoting factor that is lost during paligenosis
- PMID: 34816763
- PMCID: PMC8698547
- DOI: 10.1152/ajpgi.00246.2021
ELAPOR1 is a secretory granule maturation-promoting factor that is lost during paligenosis
Abstract
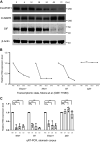
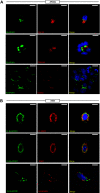
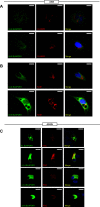
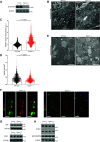
A single transcription factor, MIST1 (BHLHA15), maximizes secretory function in diverse secretory cells (like pancreatic acinar cells) by transcriptionally upregulating genes that elaborate secretory architecture. Here, we show that the scantly studied MIST1 target, ELAPOR1 (endosome/lysosome-associated apoptosis and autophagy regulator 1), is an evolutionarily conserved, novel mannose-6-phosphate receptor (M6PR) domain-containing protein. ELAPOR1 expression was specific to zymogenic cells (ZCs, the MIST1-expressing population in the stomach). ELAPOR1 expression was lost as tissue injury caused ZCs to undergo paligenosis (i.e., to become metaplastic and reenter the cell cycle). In cultured cells, ELAPOR1 trafficked with cis-Golgi resident proteins and with the trans-Golgi and late endosome protein: cation-independent M6PR. Secretory vesicle trafficking was disrupted by expression of ELAPOR1 truncation mutants. Mass spectrometric analysis of co-immunoprecipitated proteins showed ELAPOR1 and CI-M6PR shared many binding partners. However, CI-M6PR and ELAPOR1 must function differently, as CI-M6PR co-immunoprecipitated more lysosomal proteins and was not decreased during paligenosis in vivo. We generated Elapor1-/- mice to determine ELAPOR1 function in vivo. Consistent with in vitro findings, secretory granule maturation was defective in Elapor1-/- ZCs. Our results identify a role for ELAPOR1 in secretory granule maturation and help clarify how a single transcription factor maintains mature exocrine cell architecture in homeostasis and helps dismantle it during paligenosis.NEW & NOTEWORTHY Here, we find the MIST1 (BHLHA15) transcriptional target ELAPOR1 is an evolutionarily conserved, trans-Golgi/late endosome M6PR domain-containing protein that is specific to gastric zymogenic cells and required for normal secretory granule maturation in human cell lines and in mouse stomach.
Keywords: MIST1; trafficking; vesicle; zymogenic cells.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
RAB26 and RAB3D are direct transcriptional targets of MIST1 that regulate exocrine granule maturation.Mol Cell Biol. 2010 Mar;30(5):1269-84. doi: 10.1128/MCB.01328-09. Epub 2009 Dec 28. Mol Cell Biol. 2010. PMID: 20038531 Free PMC article.
-
XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum.Gastroenterology. 2010 Dec;139(6):2038-49. doi: 10.1053/j.gastro.2010.08.050. Epub 2010 Oct 14. Gastroenterology. 2010. PMID: 20816838 Free PMC article.
-
RAB26 coordinates lysosome traffic and mitochondrial localization.J Cell Sci. 2014 Mar 1;127(Pt 5):1018-32. doi: 10.1242/jcs.138776. Epub 2014 Jan 10. J Cell Sci. 2014. PMID: 24413166 Free PMC article.
-
Rab GTPases regulating receptor trafficking at the late endosome-lysosome membranes.Cell Biochem Funct. 2012 Aug;30(6):515-23. doi: 10.1002/cbf.2827. Epub 2012 Apr 3. Cell Biochem Funct. 2012. PMID: 22473705 Review.
-
Autophagy repurposes cells during paligenosis.Autophagy. 2021 Feb;17(2):588-589. doi: 10.1080/15548627.2020.1857080. Epub 2020 Dec 7. Autophagy. 2021. PMID: 33280496 Free PMC article. Review.
Cited by
-
MIST1 regulates endoplasmic reticulum stress-induced hepatic apoptosis as a candidate marker of fatty liver disease progression.Cell Death Dis. 2024 Nov 8;15(11):805. doi: 10.1038/s41419-024-07217-0. Cell Death Dis. 2024. PMID: 39516480 Free PMC article.
-
Cell plasticity in regeneration in the stomach and beyond.Curr Opin Genet Dev. 2022 Aug;75:101948. doi: 10.1016/j.gde.2022.101948. Epub 2022 Jul 6. Curr Opin Genet Dev. 2022. PMID: 35809361 Free PMC article. Review.
-
Inceptor as a regulator of brain insulin sensitivity.Sci Rep. 2023 Jul 18;13(1):11582. doi: 10.1038/s41598-023-36248-4. Sci Rep. 2023. PMID: 37463909 Free PMC article.
-
Bioinformatics Identification of Key Genes for the Development and Prognosis of Lung Adenocarcinoma.Inquiry. 2022 Jan-Dec;59:469580221096259. doi: 10.1177/00469580221096259. Inquiry. 2022. PMID: 35635202 Free PMC article.
-
A human stomach cell type transcriptome atlas.BMC Biol. 2024 Feb 14;22(1):36. doi: 10.1186/s12915-024-01812-5. BMC Biol. 2024. PMID: 38355543 Free PMC article.
References
-
- Lo H-YG, Jin RU, Sibbel G, Liu D, Karki A, Joens MS, Madison BB, Zhang B, Blanc V, Fitzpatrick JAJ, Davidson NO, Konieczny SF, Mills JC. A single transcription factor is sufficient to induce and maintain secretory cell architecture. Genes Dev 31: 154–171, 2017. doi:10.1101/gad.285684.116. - DOI - PMC - PubMed
-
- Huh WJ, Esen E, Geahlen JH, Bredemeyer AJ, Lee AH, Shi G, Konieczny SF, Glimcher LH, Mills JC. XBP1 controls maturation of gastric zymogenic cells by induction of MIST1 and expansion of the rough endoplasmic reticulum. Gastroenterology 139: 2038–2049, 2010. doi:10.1053/j.gastro.2010.08.050. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA009547/CA/NCI NIH HHS/United States
- P30 CA091842/CA/NCI NIH HHS/United States
- P41 GM103422/GM/NIGMS NIH HHS/United States
- R01 AR064793/AR/NIAMS NIH HHS/United States
- R01 DK110406/DK/NIDDK NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- R01 DK105129/DK/NIDDK NIH HHS/United States
- R01 CA239645/CA/NCI NIH HHS/United States
- R01 DK094989/DK/NIDDK NIH HHS/United States
- R24 GM136766/GM/NIGMS NIH HHS/United States
- P30 DK056338/DK/NIDDK NIH HHS/United States
- R01 CA246208/CA/NCI NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

