The double faced role of xanthine oxidoreductase in cancer
- PMID: 34811515
- PMCID: PMC9253144
- DOI: 10.1038/s41401-021-00800-7
The double faced role of xanthine oxidoreductase in cancer
Abstract
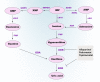
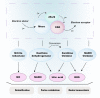
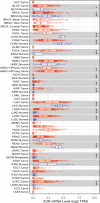
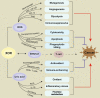
Xanthine oxidoreductase (XOR) is a critical, rate-limiting enzyme that controls the last two steps of purine catabolism by converting hypoxanthine to xanthine and xanthine to uric acid. It also produces reactive oxygen species (ROS) during the catalytic process. The enzyme is generally recognized as a drug target for the therapy of gout and hyperuricemia. The catalytic products uric acid and ROS act as antioxidants or oxidants, respectively, and are involved in pro/anti-inflammatory actions, which are associated with various disease manifestations, including metabolic syndrome, ischemia reperfusion injury, cardiovascular disorders, and cancer. Recently, extensive efforts have been devoted to understanding the paradoxical roles of XOR in tumor promotion. Here, we summarize the expression of XOR in different types of cancer and decipher the dual roles of XOR in cancer by its enzymatic or nonenzymatic activity to provide an updated understanding of the mechanistic function of XOR in cancer. We also discuss the potential to modulate XOR in cancer therapy.
Keywords: ROS; cancer therapy; uric acid; xanthine oxidoreductase (XOR).
© 2021. The Author(s), under exclusive licence to CPS and SIMM.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
New insights into purine metabolism in metabolic diseases: role of xanthine oxidoreductase activity.Am J Physiol Endocrinol Metab. 2020 Nov 1;319(5):E827-E834. doi: 10.1152/ajpendo.00378.2020. Epub 2020 Sep 7. Am J Physiol Endocrinol Metab. 2020. PMID: 32893671 Review.
-
Hyperuricemia-Related Diseases and Xanthine Oxidoreductase (XOR) Inhibitors: An Overview.Med Sci Monit. 2016 Jul 17;22:2501-12. doi: 10.12659/msm.899852. Med Sci Monit. 2016. PMID: 27423335 Free PMC article. Review.
-
Xanthine oxidoreductase: One enzyme for multiple physiological tasks.Redox Biol. 2021 May;41:101882. doi: 10.1016/j.redox.2021.101882. Epub 2021 Jan 27. Redox Biol. 2021. PMID: 33578127 Free PMC article. Review.
-
The Role of Oxidative Stress in Hyperuricemia and Xanthine Oxidoreductase (XOR) Inhibitors.Oxid Med Cell Longev. 2021 Mar 26;2021:1470380. doi: 10.1155/2021/1470380. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33854690 Free PMC article. Review.
-
Xanthine oxidoreductase: a journey from purine metabolism to cardiovascular excitation-contraction coupling.Crit Rev Biotechnol. 2011 Sep;31(3):264-80. doi: 10.3109/07388551.2010.527823. Epub 2011 Jul 20. Crit Rev Biotechnol. 2011. PMID: 21774633 Review.
Cited by
-
Exploring the causal association between uric acid and lung cancer in east Asian and European populations: a mendelian randomization study.BMC Cancer. 2024 Jul 5;24(1):801. doi: 10.1186/s12885-024-12576-0. BMC Cancer. 2024. PMID: 38965453 Free PMC article.
-
Mechanism of PDZK1 in Hepatocellular Carcinoma Complicated with Hyperuricemia.J Oncol. 2022 Nov 14;2022:1403454. doi: 10.1155/2022/1403454. eCollection 2022. J Oncol. 2022. PMID: 36420358 Free PMC article.
-
High-Accuracy Renal Cell Carcinoma Discrimination through Label-Free SERS of Blood Serum and Multivariate Analysis.Biosensors (Basel). 2023 Aug 13;13(8):813. doi: 10.3390/bios13080813. Biosensors (Basel). 2023. PMID: 37622899 Free PMC article.
-
Untargeted Metabolomic Characterization of Glioblastoma Intra-Tumor Heterogeneity Using OrbiSIMS.Anal Chem. 2023 Apr 11;95(14):5994-6001. doi: 10.1021/acs.analchem.2c05807. Epub 2023 Mar 30. Anal Chem. 2023. PMID: 36995369 Free PMC article.
-
Caffeine and Its Antioxidant Properties-It Is All about Dose and Source.Int J Mol Sci. 2022 Oct 28;23(21):13074. doi: 10.3390/ijms232113074. Int J Mol Sci. 2022. PMID: 36361861 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

