Prenatal Maternal Stress Exacerbates Experimental Colitis of Offspring in Adulthood
- PMID: 34804005
- PMCID: PMC8595204
- DOI: 10.3389/fimmu.2021.700995
Prenatal Maternal Stress Exacerbates Experimental Colitis of Offspring in Adulthood
Abstract
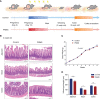
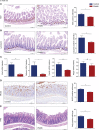
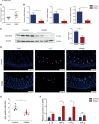
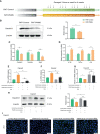
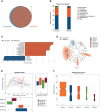
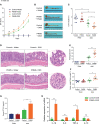
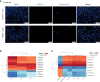
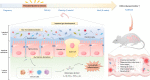
The prevalence of inflammatory bowel disease (IBD) is increasing worldwide and correlates with dysregulated immune response because of gut microbiota dysbiosis. Some adverse early life events influence the establishment of the gut microbiota and act as risk factors for IBD. Prenatal maternal stress (PNMS) induces gut dysbiosis and perturbs the neuroimmune network of offspring. In this study, we aimed to investigate whether PNMS increases the susceptibility of offspring to colitis in adulthood. The related index was assessed during the weaning period and adulthood. We found that PNMS impaired the intestinal epithelial cell proliferation, goblet cell and Paneth cell differentiation, and mucosal barrier function in 3-week-old offspring. PNMS induced low-grade intestinal inflammation, but no signs of microscopic inflammatory changes were observed. Although there was no pronounced difference between the PNMS and control offspring in terms of their overall measures of alpha diversity for the gut microbiota, distinct microbial community changes characterized by increases in Desulfovibrio, Streptococcus, and Enterococcus and decreases in Bifidobacterium and Blautia were induced in the 3-week-old PNMS offspring. Notably, the overgrowth of Desulfovibrio persisted from the weaning period to adulthood, consistent with the results observed using fluorescence in situ hybridization in the colon mucosa. Mechanistically, the fecal microbiota transplantation experiment showed that the gut microbiota from the PNMS group impaired the intestinal barrier function and induced low-grade inflammation. The fecal bacterial solution from the PNMS group was more potent than that from the control group in inducing inflammation and gut barrier disruption in CaCo-2 cells. After treatment with a TNF-α inhibitor (adalimumab), no statistical difference in the indicators of inflammation and intestinal barrier function was observed between the two groups. Finally, exposure to PNMS remarkably increased the values of the histopathological parameters and the inflammatory cytokine production in a mouse model of experimental colitis in adulthood. These findings suggest that PNMS can inhibit intestinal development, impair the barrier function, and cause gut dysbiosis characterized by the persistent overgrowth of Desulfovibrio in the offspring, resulting in exacerbated experimental colitis in adulthood.
Keywords: colitis; early life; microbiota; mucosal barrier; prenatal maternal stress.
Copyright © 2021 Sun, Xie, Li, Jin, Zhou, Huang, Li, Yang, Liu, Cao, Wang, Liu, Jiang and Cao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Prenatal Stress and Ethanol Exposure: Microbiota-Induced Immune Dysregulation and Psychiatric Risks.Int J Mol Sci. 2024 Sep 10;25(18):9776. doi: 10.3390/ijms25189776. Int J Mol Sci. 2024. PMID: 39337263 Free PMC article. Review.
-
Maternal High Fat Diet Alters Gut Microbiota of Offspring and Exacerbates DSS-Induced Colitis in Adulthood.Front Immunol. 2018 Nov 13;9:2608. doi: 10.3389/fimmu.2018.02608. eCollection 2018. Front Immunol. 2018. PMID: 30483266 Free PMC article.
-
Enteric Delivery of Regenerating Family Member 3 alpha Alters the Intestinal Microbiota and Controls Inflammation in Mice With Colitis.Gastroenterology. 2018 Mar;154(4):1009-1023.e14. doi: 10.1053/j.gastro.2017.11.003. Epub 2017 Nov 11. Gastroenterology. 2018. PMID: 29133078
-
Dihydromyricetin improves DSS-induced colitis in mice via modulation of fecal-bacteria-related bile acid metabolism.Pharmacol Res. 2021 Sep;171:105767. doi: 10.1016/j.phrs.2021.105767. Epub 2021 Jul 14. Pharmacol Res. 2021. PMID: 34273490
-
Gut bacteria signaling to mitochondria in intestinal inflammation and cancer.Gut Microbes. 2020 May 3;11(3):285-304. doi: 10.1080/19490976.2019.1592421. Epub 2019 Mar 26. Gut Microbes. 2020. PMID: 30913966 Free PMC article. Review.
Cited by
-
Prenatal Stress and Ethanol Exposure: Microbiota-Induced Immune Dysregulation and Psychiatric Risks.Int J Mol Sci. 2024 Sep 10;25(18):9776. doi: 10.3390/ijms25189776. Int J Mol Sci. 2024. PMID: 39337263 Free PMC article. Review.
-
Dysbiosis of Gut Microbiota and Intestinal Barrier Dysfunction in Pigs with Pulmonary Inflammation Induced by Mycoplasma hyorhinis Infection.mSystems. 2022 Aug 30;7(4):e0028222. doi: 10.1128/msystems.00282-22. Epub 2022 Jun 14. mSystems. 2022. PMID: 35699454 Free PMC article.
-
Long-Term Implicit Epigenetic Stress Information in the Enteric Nervous System and its Contribution to Developing and Perpetuating IBS.Curr Neuropharmacol. 2024;22(13):2100-2112. doi: 10.2174/1570159X22666240507095700. Curr Neuropharmacol. 2024. PMID: 38726788 Free PMC article. Review.
-
Efficacy of Nigella sativa seed oil against psychophysical stress induced irritable bowel syndrome and anxiety-like symptoms in Wistar rats.Psychopharmacology (Berl). 2024 Dec;241(12):2609-2626. doi: 10.1007/s00213-024-06713-7. Epub 2024 Nov 9. Psychopharmacology (Berl). 2024. PMID: 39516296
-
Contribution of hippocampal BDNF/CREB signaling pathway and gut microbiota to emotional behavior impairment induced by chronic unpredictable mild stress during pregnancy in rats offspring.PeerJ. 2022 Jun 24;10:e13605. doi: 10.7717/peerj.13605. eCollection 2022. PeerJ. 2022. PMID: 35769142 Free PMC article.
References
-
- Brzozowski B, Mazur-Bialy A, Pajdo R, Kwiecien S, Bilski J, Zwolinska-Wcislo M, et al. . Mechanisms by Which Stress Affects the Experimental and Clinical Inflammatory Bowel Disease (IBD): Role of Brain-Gut Axis. Curr Neuropharmacol (2016) 14(8):892–900. doi: 10.2174/1570159x14666160404124127 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

