Improving Deep Brain Stimulation Electrode Performance in vivo Through Use of Conductive Hydrogel Coatings
- PMID: 34803592
- PMCID: PMC8602793
- DOI: 10.3389/fnins.2021.761525
Improving Deep Brain Stimulation Electrode Performance in vivo Through Use of Conductive Hydrogel Coatings
Abstract
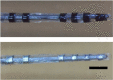
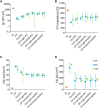
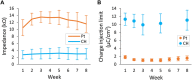
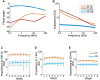
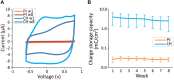
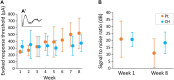
Active implantable neurological devices like deep brain stimulators have been used over the past few decades to treat movement disorders such as those in people with Parkinson's disease and more recently, in psychiatric conditions like obsessive compulsive disorder. Electrode-tissue interfaces that support safe and effective targeting of specific brain regions are critical to success of these devices. Development of directional electrodes that activate smaller volumes of brain tissue requires electrodes to operate safely with higher charge densities. Coatings such as conductive hydrogels (CHs) provide lower impedances and higher charge injection limits (CILs) than standard platinum electrodes and support safer application of smaller electrode sizes. The aim of this study was to examine the chronic in vivo performance of a new low swelling CH coating that supports higher safe charge densities than traditional platinum electrodes. A range of hydrogel blends were engineered and their swelling and electrical performance compared. Electrochemical performance and stability of high and low swelling formulations were compared during insertion into a model brain in vitro and the formulation with lower swelling characteristics was chosen for the in vivo study. CH-coated or uncoated Pt electrode arrays were implanted into the brains of 14 rats, and their electrochemical performance was tested weekly for 8 weeks. Tissue response and neural survival was assessed histologically following electrode array removal. CH coating resulted in significantly lower voltage transient impedance, higher CIL, lower electrochemical impedance spectroscopy, and higher charge storage capacity compared to uncoated Pt electrodes in vivo, and this advantage was maintained over the 8-week implantation. There was no significant difference in evoked potential thresholds, signal-to-noise ratio, tissue response or neural survival between CH-coated and uncoated Pt groups. The significant electrochemical advantage and stability of CH coating in the brain supports the suitability of this coating technology for future development of smaller, higher fidelity electrode arrays with higher charge density requirement.
Keywords: conductive hydrogel; deep brain stimulation; electrical properties; electrode coating; neural stimulation; tissue response.
Copyright © 2021 Hyakumura, Aregueta-Robles, Duan, Villalobos, Adams, Poole-Warren and Fallon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

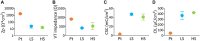

Similar articles
-
Electrochemical and biological performance of chronically stimulated conductive hydrogel electrodes.J Neural Eng. 2020 Apr 9;17(2):026018. doi: 10.1088/1741-2552/ab7cfc. J Neural Eng. 2020. PMID: 32135529
-
Electrochemical and mechanical performance of reduced graphene oxide, conductive hydrogel, and electrodeposited Pt-Ir coated electrodes: an active in vitro study.J Neural Eng. 2019 Dec 23;17(1):016015. doi: 10.1088/1741-2552/ab5163. J Neural Eng. 2019. PMID: 31652427
-
Electrochemical and biological characterization of thin-film platinum-iridium alloy electrode coatings: a chronic in vivo study.J Neural Eng. 2020 Jun 22;17(3):036012. doi: 10.1088/1741-2552/ab933d. J Neural Eng. 2020. PMID: 32408281
-
Organic electrode coatings for next-generation neural interfaces.Front Neuroeng. 2014 May 27;7:15. doi: 10.3389/fneng.2014.00015. eCollection 2014. Front Neuroeng. 2014. PMID: 24904405 Free PMC article. Review.
-
Deciphering platinum dissolution in neural stimulation electrodes: Electrochemistry or biology?Biomaterials. 2024 Sep;309:122575. doi: 10.1016/j.biomaterials.2024.122575. Epub 2024 Apr 17. Biomaterials. 2024. PMID: 38677220 Review.
Cited by
-
Cyclic Voltammetry Study of Noble Metals and Their Alloys for Use in Implantable Electrodes.ACS Omega. 2022 Sep 13;7(38):34200-34212. doi: 10.1021/acsomega.2c03563. eCollection 2022 Sep 27. ACS Omega. 2022. PMID: 36188288 Free PMC article.
-
Bioelectronic Neural Interfaces: Improving Neuromodulation Through Organic Conductive Coatings.Adv Sci (Weinh). 2024 Jul;11(27):e2306275. doi: 10.1002/advs.202306275. Epub 2023 Dec 19. Adv Sci (Weinh). 2024. PMID: 38115740 Free PMC article. Review.
-
A Comparative Study on the Effect of Substrate Structure on Electrochemical Performance and Stability of Electrodeposited Platinum and Iridium Oxide Coatings for Neural Electrodes.Micromachines (Basel). 2023 Dec 29;15(1):70. doi: 10.3390/mi15010070. Micromachines (Basel). 2023. PMID: 38258189 Free PMC article.
-
Electrochemical and biological performance of hierarchical platinum-iridium electrodes structured by a femtosecond laser.Microsyst Nanoeng. 2022 Sep 2;8:96. doi: 10.1038/s41378-022-00433-8. eCollection 2022. Microsyst Nanoeng. 2022. PMID: 36065436 Free PMC article.
-
Biohybrid neural interfaces: improving the biological integration of neural implants.Chem Commun (Camb). 2023 Dec 14;59(100):14745-14758. doi: 10.1039/d3cc05006h. Chem Commun (Camb). 2023. PMID: 37991846 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

