Progress in Nanomaterials-Based Optical and Electrochemical Methods for the Assays of Exosomes
- PMID: 34803380
- PMCID: PMC8599324
- DOI: 10.2147/IJN.S333969
Progress in Nanomaterials-Based Optical and Electrochemical Methods for the Assays of Exosomes
Abstract
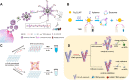
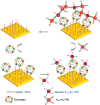
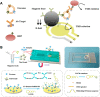
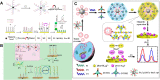
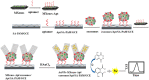
Exosomes with diameters of 30-150 nm are small membrane-bound vesicles secreted by a variety of cells. They play an important role in many biological processes, such as tumor-related immune response and intercellular signal transduction. Exosomes have been considered as emerging and noninvasive biomarkers for cancer diagnosis. Recently, a large number of optical and electrochemical biosensors have been proposed for sensitive detection of exosomes. To meet the increasing demands for ultrasensitive detection, nanomaterials have been integrated with various techniques as powerful components. Because of their intrinsic merits of biological compatibility, excellent physicochemical features and unique catalytic ability, nanomaterials have significantly improved the analytical performances of exosome biosensors. In this review, we summarized the recent progress in nanomaterials-based biosensors for the detection of cancer-derived exosomes, including fluorescence, colorimetry, surface plasmon resonance spectroscopy, surface enhanced Raman scattering spectroscopy, electrochemistry, electrochemiluminescence and so on.
Keywords: circulating tumor biomarkers; electrochemical biosensor; exosomes; nanomaterials; optical biosensor.
© 2021 Ma et al.
Conflict of interest statement
The authors declare no conflict of interest.
Figures













Similar articles
-
Recent advances in nanomaterial-based biosensors for the detection of exosomes.Anal Bioanal Chem. 2021 Jan;413(1):83-102. doi: 10.1007/s00216-020-03000-0. Epub 2020 Nov 8. Anal Bioanal Chem. 2021. PMID: 33164151 Review.
-
Recent achievements in exosomal biomarkers detection by nanomaterials-based optical biosensors - A review.Anal Chim Acta. 2020 Jun 1;1114:74-84. doi: 10.1016/j.aca.2020.02.041. Epub 2020 Feb 24. Anal Chim Acta. 2020. PMID: 32359518 Review.
-
Recent Development of Nanomaterials-Based Cytosensors for the Detection of Circulating Tumor Cells.Biosensors (Basel). 2021 Aug 18;11(8):281. doi: 10.3390/bios11080281. Biosensors (Basel). 2021. PMID: 34436082 Free PMC article. Review.
-
Electrochemical and optical biosensors based on nanomaterials and nanostructures: a review.Front Biosci (Schol Ed). 2011 Jun 1;3(4):1308-31. doi: 10.2741/228. Front Biosci (Schol Ed). 2011. PMID: 21622273 Review.
-
Progress in the research of nanomaterial-based exosome bioanalysis and exosome-based nanomaterials tumor therapy.Biomaterials. 2021 Jul;274:120873. doi: 10.1016/j.biomaterials.2021.120873. Epub 2021 May 5. Biomaterials. 2021. PMID: 33989972 Review.
Cited by
-
Exosomes as Crucial Players in Pathogenesis of Systemic Lupus Erythematosus.J Immunol Res. 2022 Jul 20;2022:8286498. doi: 10.1155/2022/8286498. eCollection 2022. J Immunol Res. 2022. PMID: 35910853 Free PMC article. Review.
-
Increasing the sensitivity and accuracy of detecting exosomes as biomarkers for cancer monitoring using optical nanobiosensors.Cancer Cell Int. 2024 May 30;24(1):189. doi: 10.1186/s12935-024-03379-1. Cancer Cell Int. 2024. PMID: 38816782 Free PMC article. Review.
-
Microfluidic Platforms for the Isolation and Detection of Exosomes: A Brief Review.Micromachines (Basel). 2022 Apr 30;13(5):730. doi: 10.3390/mi13050730. Micromachines (Basel). 2022. PMID: 35630197 Free PMC article. Review.
-
Exosomes as Powerful Biomarkers in Cancer: Recent Advances in Isolation and Detection Techniques.Int J Nanomedicine. 2024 Feb 26;19:1923-1949. doi: 10.2147/IJN.S453545. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38435755 Free PMC article. Review.
-
Graphene Sensor Arrays for Rapid and Accurate Detection of Pancreatic Cancer Exosomes in Patients' Blood Plasma Samples.ACS Nano. 2023 Aug 8;17(15):14619-14631. doi: 10.1021/acsnano.3c01812. Epub 2023 Jul 20. ACS Nano. 2023. PMID: 37470391 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

