Cancer Stem Cell Assay for the Treatment of Platinum-Resistant Recurrent Ovarian Cancer
- PMID: 34796266
- PMCID: PMC8597976
- DOI: 10.24966/srdt-2060/100076
Cancer Stem Cell Assay for the Treatment of Platinum-Resistant Recurrent Ovarian Cancer
Abstract
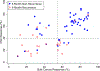
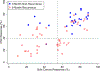
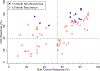
Background: Disease recurrence and progression of ovarian cancer is a common event, which is accompanied by the development of platinum-resistant or refractory disease. The presence of chemo-resistant Cancer Stem Cells (CSCs) contribute to tumor propagation, maintenance, and treatment resistance of this difficult to treat disease. We have developed ChemoID, a cytotoxic synergy assay against CSCs that identifies the most effective chemotherapy treatment from a panel of FDA-approved chemotherapies using fresh cancer biopsies.
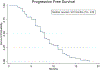
Patients and methods: Ascites or interventional radiology biopsies were collected under physician order from 78 consecutive patients affected by 3rd relapsed ovarian cancer. Test results from the assay were used when possible to treat patients with the highest cell kill drugs, taking into consideration their health status and using dose reductions, if needed. A chart analysis and review of CT and PET scans were performed to determine patients' outcomes for tumor response, Progression-Free Survival (PFS), and Overall Survival (OS).
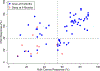
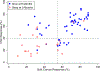
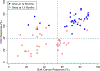
Results: We observed that recurrent ovarian cancer patients treated with high-cell kill chemotherapy agents guided by the CSCs drug response assay had an improvement in their median PFS and OS when compared to historical median PFS and OS and/or when compared to patients who could not receive high cell kill chemotherapies (PFS low cell kill 3.5 months vs. high cell kill 12.0 months; OS low cell kill 6.0 months vs. high cell kill 15.0 months).
Conclusion: This data indicates that the drug cytotoxicity assay aimed at targeting CSCs may be a useful tool for optimizing treatment selection when first-line therapy fails, and when there are multiple clinically-acceptable and -equivalent treatments available.
Keywords: Cancer stem cells; ChemoID; Chemotherapy; Ovarian cancer; Personalized medicine; Platinum resistant ovarian cancer.
Conflict of interest statement
9. Conflict of Interest Authors P.P.C. and J.V. hold intellectual property rights on the use of the ChemoID cancer stem cell assay. All other authors have no relevant disclosures to declare.
Figures




Similar articles
-
Clinical relevance of cancer stem cell chemotherapeutic assay for recurrent ovarian cancer.Transl Oncol. 2020 Dec;13(12):100860. doi: 10.1016/j.tranon.2020.100860. Epub 2020 Aug 28. Transl Oncol. 2020. PMID: 32862103 Free PMC article.
-
Analysis of Chemopredictive Assay for Targeting Cancer Stem Cells in Glioblastoma Patients.Transl Oncol. 2017 Apr;10(2):241-254. doi: 10.1016/j.tranon.2017.01.008. Epub 2017 Feb 12. Transl Oncol. 2017. PMID: 28199863 Free PMC article.
-
Cancer Stem Cell Chemotherapeutics Assay for Prospective Treatment of Recurrent Glioblastoma and Progressive Anaplastic Glioma: A Single-Institution Case Series.Transl Oncol. 2020 Apr;13(4):100755. doi: 10.1016/j.tranon.2020.100755. Epub 2020 Mar 17. Transl Oncol. 2020. PMID: 32197147 Free PMC article.
-
Poly(ADP-ribose) polymerase (PARP) inhibitors for the treatment of ovarian cancer.Cochrane Database Syst Rev. 2022 Feb 16;2(2):CD007929. doi: 10.1002/14651858.CD007929.pub4. Cochrane Database Syst Rev. 2022. PMID: 35170751 Free PMC article. Review.
-
Luteinising hormone releasing hormone (LHRH) agonists for the treatment of relapsed epithelial ovarian cancer.Cochrane Database Syst Rev. 2016 Jun 29;2016(6):CD011322. doi: 10.1002/14651858.CD011322.pub2. Cochrane Database Syst Rev. 2016. PMID: 27356090 Free PMC article. Review.
Cited by
-
Methods for Overcoming Chemoresistance in Head and Neck Squamous Cell Carcinoma: Keeping the Focus on Cancer Stem Cells, a Systematic Review.Cancers (Basel). 2024 Aug 29;16(17):3004. doi: 10.3390/cancers16173004. Cancers (Basel). 2024. PMID: 39272862 Free PMC article. Review.
-
Tumor microenvironment enriches the stemness features: the architectural event of therapy resistance and metastasis.Mol Cancer. 2022 Dec 22;21(1):225. doi: 10.1186/s12943-022-01682-x. Mol Cancer. 2022. PMID: 36550571 Free PMC article. Review.
-
Opportunities in Cancer Therapies: Deciphering the Role of Cancer Stem Cells in Tumour Repopulation.Int J Mol Sci. 2023 Dec 8;24(24):17258. doi: 10.3390/ijms242417258. Int J Mol Sci. 2023. PMID: 38139085 Free PMC article. Review.
-
The FOXO1 inhibitor AS1842856 triggers apoptosis in glioblastoma multiforme and basal-like breast cancer cells.FEBS Open Bio. 2023 Feb;13(2):352-362. doi: 10.1002/2211-5463.13547. Epub 2023 Jan 16. FEBS Open Bio. 2023. PMID: 36602390 Free PMC article.
References
-
- Hutchinson L, Romero D (2016) Precision or imprecision medicine? Nat Rev Clin Oncol 13: 713. - PubMed
-
- Deng X, Nakamura Y (2017) Cancer Precision Medicine: From Cancer Screening to Drug Selection and Personalized Immunotherapy. Trends Pharmacol Sci 38: 15–24. - PubMed
-
- Millner LM, Strotman LN (2016) The Future of Precision Medicine in Oncology. Clin Lab Med 36: 557–573. - PubMed
-
- Prasad V (2016) Perspective: The precision-oncology illusion. Nature 537: S63. - PubMed
-
- Maenpaa JU, Heinonen E, Hinkka SM, Karnani P, Klemi PJ, et al. (1995) The subrenal capsule assay in selecting chemotherapy for ovarian cancer: a prospective randomized trial. Gynecol Oncol 57: 294–298. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
