Butyric Acid Protects Against Renal Ischemia-Reperfusion Injury by Adjusting the Treg/Th17 Balance via HO-1/p-STAT3 Signaling
- PMID: 34796171
- PMCID: PMC8593469
- DOI: 10.3389/fcell.2021.733308
Butyric Acid Protects Against Renal Ischemia-Reperfusion Injury by Adjusting the Treg/Th17 Balance via HO-1/p-STAT3 Signaling
Erratum in
-
Corrigendum: Butyric acid protects against renal ischemia-reperfusion injury by adjusting the Treg/Th17 balance via HO-1/p-STAT3 signaling.Front Cell Dev Biol. 2022 Aug 24;10:999965. doi: 10.3389/fcell.2022.999965. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36092696 Free PMC article.
Abstract
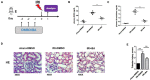
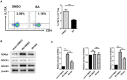
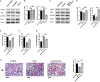
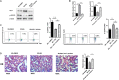
Immune regulation plays a vital role in ischemia-reperfusion injury (IRI). Butyric acid (BA) has immunomodulatory effects in many diseases, but its immunomodulatory effects during renal IRI are still unclear. Our research shows that BA protected against IRI and significantly improved renal IRI in vivo. In vitro studies showed that BA inhibits Th17 cell differentiation and induces Treg cell differentiation. Mechanism studies have shown that heme oxygenase 1 (HO-1)/STAT3 signaling pathway was involved in the inhibitory effect of BA on Th17 cell differentiation. HO-1 inhibitors can significantly rescue the BA-mediated inhibition of Th17 cell differentiation. We confirmed that BA promotes the differentiation of Th17 cells into Treg cells by regulating the pathway and reduces renal IRI.
Keywords: HO-1; STAT3; Th17; Treg; butyric acid; renal ischemia–reperfusion injury.
Copyright © 2021 Chen, Wang, Yang, Shi, Ji, Ding, Jiang, Fan, Chen and Lu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Corrigendum: Butyric acid protects against renal ischemia-reperfusion injury by adjusting the Treg/Th17 balance via HO-1/p-STAT3 signaling.Front Cell Dev Biol. 2022 Aug 24;10:999965. doi: 10.3389/fcell.2022.999965. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36092696 Free PMC article.
-
Gal-9/Tim-3 signaling pathway activation suppresses the generation of Th17 cells and promotes the induction of Foxp3+ regulatory T cells in renal ischemia-reperfusion injury.Mol Immunol. 2023 Apr;156:136-147. doi: 10.1016/j.molimm.2023.03.008. Epub 2023 Mar 13. Mol Immunol. 2023. PMID: 36921488
-
Transcriptional modulation of the T helper 17/interleukin 17 axis ameliorates renal ischemia-reperfusion injury.Nephrol Dial Transplant. 2019 Sep 1;34(9):1481-1498. doi: 10.1093/ndt/gfy370. Nephrol Dial Transplant. 2019. PMID: 30544214
-
Deciphering the Role of Heme Oxygenase-1 (HO-1) Expressing Macrophages in Renal Ischemia-Reperfusion Injury.Biomedicines. 2021 Mar 16;9(3):306. doi: 10.3390/biomedicines9030306. Biomedicines. 2021. PMID: 33809696 Free PMC article. Review.
-
Mesenchymal Stem Cell Protects Injured Renal Tubular Epithelial Cells by Regulating mTOR-Mediated Th17/Treg Axis.Front Immunol. 2021 May 28;12:684197. doi: 10.3389/fimmu.2021.684197. eCollection 2021. Front Immunol. 2021. PMID: 34122446 Free PMC article. Review.
Cited by
-
A molecular network-based pharmacological study on the protective effect of Panax notoginseng rhizomes against renal ischemia-reperfusion injury.Front Pharmacol. 2023 Apr 18;14:1134408. doi: 10.3389/fphar.2023.1134408. eCollection 2023. Front Pharmacol. 2023. PMID: 37144215 Free PMC article.
-
Clostridium butyricum inhibits the inflammation in children with primary nephrotic syndrome by regulating Th17/Tregs balance via gut-kidney axis.BMC Microbiol. 2024 Mar 23;24(1):97. doi: 10.1186/s12866-024-03242-3. BMC Microbiol. 2024. PMID: 38521894 Free PMC article.
-
Heme Oxygenase-1 Expression in Dendritic Cells Contributes to Protective Immunity against Herpes Simplex Virus Skin Infection.Antioxidants (Basel). 2023 May 29;12(6):1170. doi: 10.3390/antiox12061170. Antioxidants (Basel). 2023. PMID: 37371900 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

