Lipid Nanoparticle-mRNA Formulations for Therapeutic Applications
- PMID: 34793124
- PMCID: PMC10068911
- DOI: 10.1021/acs.accounts.1c00550
Lipid Nanoparticle-mRNA Formulations for Therapeutic Applications
Abstract
After decades of extensive fundamental studies and clinical trials, lipid nanoparticles (LNPs) have demonstrated effective mRNA delivery such as the Moderna and Pfizer-BioNTech vaccines fighting against COVID-19. Moreover, researchers and clinicians have been investigating mRNA therapeutics for a variety of therapeutic indications including protein replacement therapy, genome editing, and cancer immunotherapy. To realize these therapeutics in the clinic, there are many formidable challenges. First, novel delivery systems such as LNPs with high delivery efficiency and low toxicity need to be developed for different cell types. Second, mRNA molecules need to be engineered for improved pharmaceutical properties. Lastly, the LNP-mRNA nanoparticle formulations need to match their therapeutic applications.In this Account, we summarize our recent advances in the design and development of various classes of lipids and lipid derivatives, which can be formulated with multiple types of mRNA molecules to treat diverse diseases. For example, we conceived a series of ionizable lipid-like molecules based on the structures of a benzene core, an amide linker, and hydrophobic tails. We identified N1,N3,N5-tris(3-(didodecylamino)propyl)benzene-1,3,5-tricarboxamide (TT3) as a lead compound for mRNA delivery both in vitro and in vivo. Moreover, we tuned the biodegradability of these lipid-like molecules by introducing branched ester or linear ester chains. Meanwhile, inspired by biomimetic compounds, we synthesized vitamin-derived lipids, chemotherapeutic conjugated lipids, phospholipids, and glycolipids. These scaffolds greatly broaden the chemical space of ionizable lipids for mRNA delivery. In another section, we highlight our efforts on the research direction of mRNA engineering. We previously optimized mRNA chemistry using chemically-modified nucleotides to increase the protein expression, such as pseudouridine (ψ), 5-methoxyuridine (5moU), and N1-methylpseudouridine (me1ψ). Also, we engineered the sequences of mRNA 5' untranslated regions (5'-UTRs) and 3' untranslated regions (3'-UTRs), which dramatically enhanced protein expression. With the progress of LNP development and mRNA engineering, we consolidate these technologies and apply them to treat diseases such as genetic disorders, infectious diseases, and cancers. For instance, TT3 and its analog-derived lipid-like nanoparticles can effectively deliver factor IX or VIII mRNA and recover the clotting activity in hemophilia mouse models. Engineered mRNAs encoding SARS-CoV-2 antigens serve well as vaccine candidates against COVID-19. Vitamin-derived lipid nanoparticles loaded with antimicrobial peptide-cathepsin B mRNA enable adoptive macrophage transfer to treat multidrug resistant bacterial sepsis. Biomimetic lipids such as phospholipids formulated with mRNAs encoding costimulatory receptors lead to enhanced cancer immunotherapy.Overall, lipid-mRNA nanoparticle formulations have considerably benefited public health in the COVID-19 pandemic. To expand their applications in clinical use, research work from many disciplines such as chemistry, engineering, materials, pharmaceutical sciences, and medicine need to be integrated. With these collaborative efforts, we believe that more and more lipid-mRNA nanoparticle formulations will enter the clinic in the near future and benefit human health.
Conflict of interest statement
The authors declare the following competing financial interest(s): C.W. and Y.Z. declare no competing interests. Y.D. is a scientific advisory board member of Oncorus, Inc.
Figures

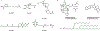

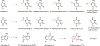
Similar articles
-
Developing Biodegradable Lipid Nanoparticles for Intracellular mRNA Delivery and Genome Editing.Acc Chem Res. 2021 Nov 2;54(21):4001-4011. doi: 10.1021/acs.accounts.1c00500. Epub 2021 Oct 20. Acc Chem Res. 2021. PMID: 34668716 Review.
-
From influenza to COVID-19: Lipid nanoparticle mRNA vaccines at the frontiers of infectious diseases.Acta Biomater. 2021 Sep 1;131:16-40. doi: 10.1016/j.actbio.2021.06.023. Epub 2021 Jun 18. Acta Biomater. 2021. PMID: 34153512 Free PMC article. Review.
-
Chemistry of Lipid Nanoparticles for RNA Delivery.Acc Chem Res. 2022 Jan 4;55(1):2-12. doi: 10.1021/acs.accounts.1c00544. Epub 2021 Dec 1. Acc Chem Res. 2022. PMID: 34850635 Review.
-
Enzyme-Catalyzed One-Step Synthesis of Ionizable Cationic Lipids for Lipid Nanoparticle-Based mRNA COVID-19 Vaccines.ACS Nano. 2022 Nov 22;16(11):18936-18950. doi: 10.1021/acsnano.2c07822. Epub 2022 Oct 21. ACS Nano. 2022. PMID: 36269150
-
Lipid nanoparticle-based mRNA candidates elicit potent T cell responses.Biomater Sci. 2023 Jan 31;11(3):964-974. doi: 10.1039/d2bm01581a. Biomater Sci. 2023. PMID: 36537916
Cited by
-
Delivery of RNAs to Specific Organs by Lipid Nanoparticles for Gene Therapy.Pharmaceutics. 2022 Oct 7;14(10):2129. doi: 10.3390/pharmaceutics14102129. Pharmaceutics. 2022. PMID: 36297564 Free PMC article. Review.
-
Carrier strategies boost the application of CRISPR/Cas system in gene therapy.Exploration (Beijing). 2022 Mar 15;2(2):20210081. doi: 10.1002/EXP.20210081. eCollection 2022 Apr. Exploration (Beijing). 2022. PMID: 37323878 Free PMC article. Review.
-
Cardiac resident macrophages: Spatiotemporal distribution, development, physiological functions, and their translational potential on cardiac diseases.Acta Pharm Sin B. 2024 Apr;14(4):1483-1493. doi: 10.1016/j.apsb.2023.12.018. Epub 2024 Jan 2. Acta Pharm Sin B. 2024. PMID: 38572111 Free PMC article. Review.
-
The therapeutic prospects of N-acetylgalactosamine-siRNA conjugates.Front Pharmacol. 2022 Dec 14;13:1090237. doi: 10.3389/fphar.2022.1090237. eCollection 2022. Front Pharmacol. 2022. PMID: 36588695 Free PMC article. Review.
-
Intratumoural Delivery of mRNA Loaded on a Cationic Hyper-Branched Cyclodextrin-Based Polymer Induced an Anti-Tumour Immunological Response in Melanoma.Cancers (Basel). 2023 Jul 24;15(14):3748. doi: 10.3390/cancers15143748. Cancers (Basel). 2023. PMID: 37509409 Free PMC article.
References
-
-
Li B; Luo X; Deng B; Wang J; McComb DW; Shi Y; Gaensler KML; Tan X; Dunn AL; Kerlin BA; Dong Y An Orthogonal Array Optimization of Lipid-Like Nanoparticles for mRNA Delivery in Vivo. Nano Lett. 2015, 15, 8099–8107.
This paper reported the development of TT3 lipid-like nanoparticles for mRNA delivery in a hemophilia B mouse model.
-
-
-
Zhang X; Zhao W; Nguyen GN; Zhang C; Zeng C; Yan J; Du S; Hou X; Li W; Jiang J; Deng B; McComb DW; Dorkin R; Shah A; Barrera L; Gregoire F; Singh M; Chen D; Sabatino DE; Dong Y Functionalized Lipid-Like Nanoparticles for in Vivo mRNA Delivery and Base Editing. Sci. Adv. 2020, 6, No. eabc2315.
This paper reported the chemical modifications of lipid-like molecules. The lead material FTT5 LLNs effectively delivered human factor VIII mRNA and base editing components in mouse models.
-
-
-
Zeng C; Hou X; Yan J; Zhang C; Li W; Zhao W; Du S; Dong Y Leveraging mRNA Sequences and Nanoparticles to Deliver SARS-CoV-2 Antigens in Vivo. Adv. Mater. 2020, 32, 2004452.
This paper reported the approaches for engineering mRNA UTR and applied the lead UTR for constructing COVID-19 mRNA vaccine candidates.
-
-
-
Hou X; Zhang X; Zhao W; Zeng C; Deng B; McComb DW; Du S; Zhang C; Li W; Dong Y Vitamin Lipid Nanoparticles Enable Adoptive Macrophage Transfer for the Treatment of Multidrug-Resistant Bacterial Sepsis. Nat. Nanotechnol. 2020, 15, 41–46.
This paper reported the development of vitamin C lipid nanoparticle (VcLNP) and applied the VcLNP–mRNA formulation to engineer macrophages for the treatment of multidrug-resistant bacterial sepsis.
-
-
- Brenner S; Jacob F; Meselson M An Unstable Intermediate Carrying Information from Genes to Ribosomes for Protein Synthesis. Nature 1961, 190, 576–581. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

