SOX transcription factors and glioma stem cells: Choosing between stemness and differentiation
- PMID: 34786152
- PMCID: PMC8567447
- DOI: 10.4252/wjsc.v13.i10.1417
SOX transcription factors and glioma stem cells: Choosing between stemness and differentiation
Abstract
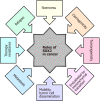
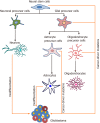
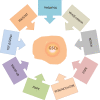
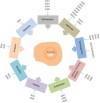
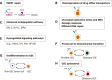
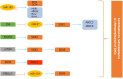
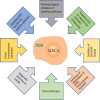
Glioblastoma (GBM) is the most common, most aggressive and deadliest brain tumor. Recently, remarkable progress has been made towards understanding the cellular and molecular biology of gliomas. GBM tumor initiation, progression and relapse as well as resistance to treatments are associated with glioma stem cells (GSCs). GSCs exhibit a high proliferation rate and self-renewal capacity and the ability to differentiate into diverse cell types, generating a range of distinct cell types within the tumor, leading to cellular heterogeneity. GBM tumors may contain different subsets of GSCs, and some of them may adopt a quiescent state that protects them against chemotherapy and radiotherapy. GSCs enriched in recurrent gliomas acquire more aggressive and therapy-resistant properties, making them more malignant, able to rapidly spread. The impact of SOX transcription factors (TFs) on brain tumors has been extensively studied in the last decade. Almost all SOX genes are expressed in GBM, and their expression levels are associated with patient prognosis and survival. Numerous SOX TFs are involved in the maintenance of the stemness of GSCs or play a role in the initiation of GSC differentiation. The fine-tuning of SOX gene expression levels controls the balance between cell stemness and differentiation. Therefore, innovative therapies targeting SOX TFs are emerging as promising tools for combatting GBM. Combatting GBM has been a demanding and challenging goal for decades. The current therapeutic strategies have not yet provided a cure for GBM and have only resulted in a slight improvement in patient survival. Novel approaches will require the fine adjustment of multimodal therapeutic strategies that simultaneously target numerous hallmarks of cancer cells to win the battle against GBM.
Keywords: Differentiation; Glioblastoma; Glioma stem cells; SOX transcription factors; Stemness.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interest for this article.
Figures

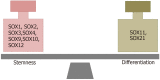
Similar articles
-
Different states of stemness of glioblastoma stem cells sustain glioblastoma subtypes indicating novel clinical biomarkers and high-efficacy customized therapies.J Exp Clin Cancer Res. 2023 Sep 21;42(1):244. doi: 10.1186/s13046-023-02811-0. J Exp Clin Cancer Res. 2023. PMID: 37735434 Free PMC article.
-
Stemness Marker Detection in the Periphery of Glioblastoma and Ability of Glioblastoma to Generate Glioma Stem Cells: Clinical Correlations.World Neurosurg. 2017 Sep;105:895-905. doi: 10.1016/j.wneu.2017.05.099. Epub 2017 May 27. World Neurosurg. 2017. PMID: 28559081
-
High expression of RFX4 is associated with tumor progression and poor prognosis in patients with glioblastoma.Int J Neurosci. 2021 Jan;131(1):7-14. doi: 10.1080/00207454.2020.1732969. Epub 2020 Mar 2. Int J Neurosci. 2021. PMID: 32075484
-
Mitochondria's Role in the Maintenance of Cancer Stem Cells in Glioblastoma.Front Oncol. 2021 Feb 22;11:582694. doi: 10.3389/fonc.2021.582694. eCollection 2021. Front Oncol. 2021. PMID: 33692947 Free PMC article. Review.
-
Emerging Role of Glioma Stem Cells in Mechanisms of Therapy Resistance.Cancers (Basel). 2023 Jul 1;15(13):3458. doi: 10.3390/cancers15133458. Cancers (Basel). 2023. PMID: 37444568 Free PMC article. Review.
Cited by
-
Regulatory Network of Methyltransferase-Like 3 in Stem Cells: Mechanisms and Medical Implications.Cell Transplant. 2024 Jan-Dec;33:9636897241282792. doi: 10.1177/09636897241282792. Cell Transplant. 2024. PMID: 39466679 Free PMC article. Review.
-
Revealing the role of SPP1+ macrophages in glioma prognosis and therapeutic targeting by investigating tumor-associated macrophage landscape in grade 2 and 3 gliomas.Cell Biosci. 2024 Mar 21;14(1):37. doi: 10.1186/s13578-024-01218-4. Cell Biosci. 2024. PMID: 38515213 Free PMC article.
-
Efficient Inference of Spatially-Varying Gaussian Markov Random Fields With Applications in Gene Regulatory Networks.IEEE/ACM Trans Comput Biol Bioinform. 2023 Sep-Oct;20(5):2920-2932. doi: 10.1109/TCBB.2023.3282028. Epub 2023 Oct 9. IEEE/ACM Trans Comput Biol Bioinform. 2023. PMID: 37276119 Free PMC article.
-
The Role of SOX Transcription Factors in Ageing and Age-Related Diseases.Int J Mol Sci. 2023 Jan 3;24(1):851. doi: 10.3390/ijms24010851. Int J Mol Sci. 2023. PMID: 36614288 Free PMC article. Review.
-
Machine learning-based identification of SOX10 as an immune regulator of macrophage in gliomas.Front Immunol. 2022 Nov 29;13:1007461. doi: 10.3389/fimmu.2022.1007461. eCollection 2022. Front Immunol. 2022. PMID: 36524115 Free PMC article.
References
-
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. - PubMed
-
- Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability--an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–228. - PubMed
-
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

