International alliance and AGREE-ment of 71 clinical practice guidelines on the management of critical care patients with COVID-19: a living systematic review
- PMID: 34785346
- PMCID: PMC8590623
- DOI: 10.1016/j.jclinepi.2021.11.010
International alliance and AGREE-ment of 71 clinical practice guidelines on the management of critical care patients with COVID-19: a living systematic review
Abstract
Objective: We aimed to systematically identify and critically assess the clinical practice guidelines (CPGs) for the management of critically ill patients with COVID-19 with the AGREE II instrument.
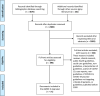
Study design and setting: We searched Medline, CINAHL, EMBASE, CNKI, CBM, WanFang, and grey literature from November 2019 - November 2020. We did not apply language restrictions. One reviewer independently screened the retrieved titles and abstracts, and a second reviewer confirmed the decisions. Full texts were assessed independently and in duplicate. Disagreements were resolved by consensus. We included any guideline that provided recommendations on the management of critically ill patients with COVID-19. Data extraction was performed independently and in duplicate by two reviewers. We descriptively summarized CPGs characteristics. We assessed the quality with the AGREE II instrument and we summarized relevant therapeutic interventions.
Results: We retrieved 3,907 records and 71 CPGs were included. Means (Standard Deviations) of the scores for the 6 domains of the AGREE II instrument were 65%(SD19.56%), 39%(SD19.64%), 27%(SD19.48%), 70%(SD15.74%), 26%(SD18.49%), 42%(SD34.91) for the scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, editorial independence domains, respectively. Most of the CPGs showed a low overall quality (less than 40%).
Conclusion: Future CPGs for COVID-19 need to rely, for their development, on standard evidence-based methods and tools.
Keywords: AGREE II tool; COVID-19; guidelines; intensive care; quality; rapid guidelines.
Copyright © 2021 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Quality appraisal of clinical practice guidelines addressing massage interventions using the AGREE II instrument.Syst Rev. 2024 Mar 8;13(1):83. doi: 10.1186/s13643-024-02503-6. Syst Rev. 2024. PMID: 38459534 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Screening for osteoporosis: A systematic assessment of the quality and content of clinical practice guidelines, using the AGREE II instrument and the IOM Standards for Trustworthy Guidelines.PLoS One. 2018 Dec 6;13(12):e0208251. doi: 10.1371/journal.pone.0208251. eCollection 2018. PLoS One. 2018. PMID: 30521556 Free PMC article.
-
Clinical practice guidelines for the treatment and management of low back pain: A systematic review of quantity and quality.Musculoskelet Sci Pract. 2021 Feb;51:102295. doi: 10.1016/j.msksp.2020.102295. Epub 2020 Nov 7. Musculoskelet Sci Pract. 2021. PMID: 33444892 Review.
-
Quality Assessment of the Clinical Practice Guidelines of Ostomy Care Based on the AGREE II Instrument.Front Public Health. 2022 Jul 4;10:856325. doi: 10.3389/fpubh.2022.856325. eCollection 2022. Front Public Health. 2022. PMID: 35859774 Free PMC article. Review.
Cited by
-
Methodological quality and reporting quality of COVID-19 living systematic review: a cross-sectional study.BMC Med Res Methodol. 2023 Jul 31;23(1):175. doi: 10.1186/s12874-023-01980-y. BMC Med Res Methodol. 2023. PMID: 37525117 Free PMC article.
-
A living critical interpretive synthesis to yield a framework on the production and dissemination of living evidence syntheses for decision-making.Implement Sci. 2024 Sep 27;19(1):67. doi: 10.1186/s13012-024-01396-2. Implement Sci. 2024. PMID: 39334425 Free PMC article. Review.
-
Searching for evidence in public health emergencies: a white paper of best practices.J Med Libr Assoc. 2023 Apr 21;111(1-2):566-578. doi: 10.5195/jmla.2023.1530. J Med Libr Assoc. 2023. PMID: 37312802 Free PMC article.
-
Characteristics of Living Systematic Review for COVID-19.Clin Epidemiol. 2022 Aug 4;14:925-935. doi: 10.2147/CLEP.S367339. eCollection 2022. Clin Epidemiol. 2022. PMID: 35958161 Free PMC article.
-
Involvement of methodological experts and the quality of clinical practice guidelines: a critical appraisal of clinical practice guidelines and a questionnaire survey of the development groups in Japan.BMJ Open. 2023 May 15;13(5):e063639. doi: 10.1136/bmjopen-2022-063639. BMJ Open. 2023. PMID: 37188477 Free PMC article.
References
-
- WHO Director-General's opening remarks at the media briefing on COVID-19 - 11 March 2020 [Internet]. Who.int. 2020 [cited 6 April 2020]. Available at: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-re...
-
- Covid19.who.int. 2020. WHO Coronavirus Disease (COVID-19) Dashboard. [online] Available at: <https://covid19.who.int/> [Accessed 15 Feb 2021].
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical