daf-16/FOXO blocks adult cell fate in Caenorhabditis elegans dauer larvae via lin-41/TRIM71
- PMID: 34780472
- PMCID: PMC8629381
- DOI: 10.1371/journal.pgen.1009881
daf-16/FOXO blocks adult cell fate in Caenorhabditis elegans dauer larvae via lin-41/TRIM71
Abstract
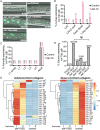
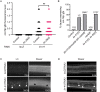
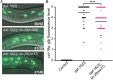
Many tissue-specific stem cells maintain the ability to produce multiple cell types during long periods of non-division, or quiescence. FOXO transcription factors promote quiescence and stem cell maintenance, but the mechanisms by which FOXO proteins promote multipotency during quiescence are still emerging. The single FOXO ortholog in C. elegans, daf-16, promotes entry into a quiescent and stress-resistant larval stage called dauer in response to adverse environmental cues. During dauer, stem and progenitor cells maintain or re-establish multipotency to allow normal development to resume after dauer. We find that during dauer, daf-16/FOXO prevents epidermal stem cells (seam cells) from prematurely adopting differentiated, adult characteristics. In particular, dauer larvae that lack daf-16 misexpress collagens that are normally adult-enriched. Using col-19p::gfp as an adult cell fate marker, we find that all major daf-16 isoforms contribute to opposing col-19p::gfp expression during dauer. By contrast, daf-16(0) larvae that undergo non-dauer development do not misexpress col-19p::gfp. Adult cell fate and the timing of col-19p::gfp expression are regulated by the heterochronic gene network, including lin-41 and lin-29. lin-41 encodes an RNA-binding protein orthologous to LIN41/TRIM71 in mammals, and lin-29 encodes a conserved zinc finger transcription factor. In non-dauer development, lin-41 opposes adult cell fate by inhibiting the translation of lin-29, which directly activates col-19 transcription and promotes adult cell fate. We find that during dauer, lin-41 blocks col-19p::gfp expression, but surprisingly, lin-29 is not required in this context. Additionally, daf-16 promotes the expression of lin-41 in dauer larvae. The col-19p::gfp misexpression phenotype observed in dauer larvae with reduced daf-16 requires the downregulation of lin-41, but does not require lin-29. Taken together, this work demonstrates a novel role for daf-16/FOXO as a heterochronic gene that promotes expression of lin-41/TRIM71 to contribute to multipotent cell fate in a quiescent stem cell model.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Control of cell-fate plasticity and maintenance of multipotency by DAF-16/FoxO in quiescent Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2013 Feb 5;110(6):2181-6. doi: 10.1073/pnas.1222377110. Epub 2013 Jan 22. Proc Natl Acad Sci U S A. 2013. PMID: 23341633 Free PMC article.
-
Unexpected role for dosage compensation in the control of dauer arrest, insulin-like signaling, and FoxO transcription factor activity in Caenorhabditis elegans.Genetics. 2013 Jul;194(3):619-29. doi: 10.1534/genetics.113.149948. Epub 2013 Jun 3. Genetics. 2013. PMID: 23733789 Free PMC article.
-
daf-31 encodes the catalytic subunit of N alpha-acetyltransferase that regulates Caenorhabditis elegans development, metabolism and adult lifespan.PLoS Genet. 2014 Oct 16;10(10):e1004699. doi: 10.1371/journal.pgen.1004699. eCollection 2014 Oct. PLoS Genet. 2014. PMID: 25330189 Free PMC article.
-
Integration of diverse inputs in the regulation of Caenorhabditis elegans DAF-16/FOXO.Dev Dyn. 2010 May;239(5):1405-12. doi: 10.1002/dvdy.22244. Dev Dyn. 2010. PMID: 20140911 Free PMC article. Review.
-
The search for DAF-16/FOXO transcriptional targets: approaches and discoveries.Exp Gerontol. 2006 Oct;41(10):910-21. doi: 10.1016/j.exger.2006.06.040. Epub 2006 Aug 24. Exp Gerontol. 2006. PMID: 16934425 Review.
Cited by
-
ets-10p::gfp expression is predictive of dauer formation in daf-16; daf-7 larvae.MicroPubl Biol. 2024 Oct 22;2024:10.17912/micropub.biology.001358. doi: 10.17912/micropub.biology.001358. eCollection 2024. MicroPubl Biol. 2024. PMID: 39502419 Free PMC article.
-
Transcriptional and spatiotemporal regulation of the dauer program.Transcription. 2023 Nov;14(1-2):27-48. doi: 10.1080/21541264.2023.2190295. Epub 2023 Mar 23. Transcription. 2023. PMID: 36951297 Free PMC article. Review.
-
Forkhead box O proteins: steering the course of stem cell fate.Cell Regen. 2024 Mar 11;13(1):7. doi: 10.1186/s13619-024-00190-1. Cell Regen. 2024. PMID: 38466341 Free PMC article. Review.
-
Dauer larva-derived extracellular vesicles extend the life of Caenorhabditis elegans.Biogerontology. 2023 Aug;24(4):581-592. doi: 10.1007/s10522-023-10030-5. Epub 2023 Apr 13. Biogerontology. 2023. PMID: 37052773 Free PMC article.
-
Harnessing full-text publications for deep insights into C. elegans and Drosophila biomaps.BMC Genomics. 2024 Nov 13;25(1):1080. doi: 10.1186/s12864-024-10997-6. BMC Genomics. 2024. PMID: 39538127 Free PMC article.
References
-
- Euling S, Ambros V. Reversal of cell fate determination in Caenorhabditis elegans vulval development. Development. 1996;122: 2507–2515. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

