Making a Killer: Selecting the Optimal Natural Killer Cells for Improved Immunotherapies
- PMID: 34777383
- PMCID: PMC8578927
- DOI: 10.3389/fimmu.2021.765705
Making a Killer: Selecting the Optimal Natural Killer Cells for Improved Immunotherapies
Abstract
Over the past 20 years natural killer (NK) cell-based immunotherapies have emerged as a safe and effective treatment option for patients with relapsed or refractory leukemia. Unlike T cell-based therapies, NK cells harbor an innate capacity to eliminate malignant cells without prior sensitization and can be adoptively transferred between individuals without the need for extensive HLA matching. A wide variety of therapeutic NK cell sources are currently being investigated clinically, including allogeneic donor-derived NK cells, stem cell-derived NK cells and NK cell lines. However, it is becoming increasingly clear that not all NK cells are endowed with the same antitumor potential. Despite advances in techniques to enhance NK cell cytotoxicity and persistence, the initial identification and utilization of highly functional NK cells remains essential to ensure the future success of adoptive NK cell therapies. Indeed, little consideration has been given to the identification and selection of donors who harbor NK cells with potent antitumor activity. In this regard, there is currently no standard donor selection criteria for adoptive NK cell therapy. Here, we review our current understanding of the factors which govern NK cell functional fate, and propose a paradigm shift away from traditional phenotypic characterization of NK cell subsets towards a functional profile based on molecular and metabolic characteristics. We also discuss previous selection models for NK cell-based immunotherapies and highlight important considerations for the selection of optimal NK cell donors for future adoptive cell therapies.
Keywords: cancer immunotherapy; cell metabolism; donor selection; epigenetics; natural killer cells; phenotype; transcriptomics.
Copyright © 2021 Barnes, Trew, de Jong and Foley.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

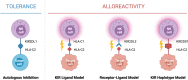
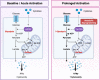
Similar articles
-
An Uncoupling of Canonical Phenotypic Markers and Functional Potency of Ex Vivo-Expanded Natural Killer Cells.Front Immunol. 2018 Feb 2;9:150. doi: 10.3389/fimmu.2018.00150. eCollection 2018. Front Immunol. 2018. PMID: 29456538 Free PMC article.
-
Natural Killer Cells: The Linchpin for Successful Cancer Immunotherapy.Front Immunol. 2021 Apr 28;12:679117. doi: 10.3389/fimmu.2021.679117. eCollection 2021. Front Immunol. 2021. PMID: 33995422 Free PMC article. Review.
-
Natural killer (NK) cell-based immunotherapies and the many faces of NK cell memory: A look into how nanoparticles enhance NK cell activity.Adv Drug Deliv Rev. 2021 Sep;176:113860. doi: 10.1016/j.addr.2021.113860. Epub 2021 Jul 5. Adv Drug Deliv Rev. 2021. PMID: 34237404 Review.
-
Prospects and Advances in Adoptive Natural Killer Cell Therapy for Unmet Therapeutic Needs in Pediatric Bone Sarcomas.Int J Mol Sci. 2023 May 5;24(9):8324. doi: 10.3390/ijms24098324. Int J Mol Sci. 2023. PMID: 37176035 Free PMC article. Review.
-
Improving natural killer cell cancer immunotherapy.Curr Opin Organ Transplant. 2015 Dec;20(6):671-80. doi: 10.1097/MOT.0000000000000243. Curr Opin Organ Transplant. 2015. PMID: 26414502 Free PMC article. Review.
Cited by
-
Transcriptional rewiring in CD8+ T cells: implications for CAR-T cell therapy against solid tumours.Front Immunol. 2024 Sep 27;15:1412731. doi: 10.3389/fimmu.2024.1412731. eCollection 2024. Front Immunol. 2024. PMID: 39399500 Free PMC article. Review.
-
Bulk and single-cell transcriptomics identify gene signatures of stem cell-derived NK cell donors with superior cytolytic activity.Mol Ther Oncol. 2024 Sep 2;32(4):200870. doi: 10.1016/j.omton.2024.200870. eCollection 2024 Dec 19. Mol Ther Oncol. 2024. PMID: 39346765 Free PMC article.
-
Genetic Manipulation Approaches to Enhance the Clinical Application of NK Cell-Based Immunotherapy.Stem Cells Transl Med. 2024 Mar 15;13(3):230-242. doi: 10.1093/stcltm/szad087. Stem Cells Transl Med. 2024. PMID: 38142460 Free PMC article. Review.
-
Building a Better Defense: Expanding and Improving Natural Killer Cells for Adoptive Cell Therapy.Cells. 2024 Mar 5;13(5):451. doi: 10.3390/cells13050451. Cells. 2024. PMID: 38474415 Free PMC article. Review.
-
CAR-NK Cell Therapy: A Transformative Approach to Overcoming Oncological Challenges.Biomolecules. 2024 Aug 20;14(8):1035. doi: 10.3390/biom14081035. Biomolecules. 2024. PMID: 39199421 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

