Excitatory Amino Acid Transporter EAAT5 Improves Temporal Resolution in the Retina
- PMID: 34772693
- PMCID: PMC8670604
- DOI: 10.1523/ENEURO.0406-21.2021
Excitatory Amino Acid Transporter EAAT5 Improves Temporal Resolution in the Retina
Abstract
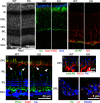
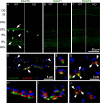
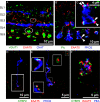
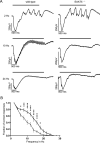
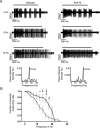
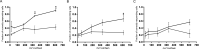
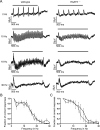
Excitatory amino acid transporters (EAATs) remove glutamate from the synaptic cleft. In the retina, EAAT1 and EAAT2 are considered the major glutamate transporters. However, it has not yet been possible to determine how EAAT5 shapes the retinal light responses because of the lack of a selective EAAT5 blocker or EAAT5 knock-out (KO) animal model. In this study, EAAT5 was found to be expressed in a punctate manner close to release sites of glutamatergic synapses in the mouse retina. Light responses from retinae of wild-type (WT) and of a newly generated model with a targeted deletion of EAAT5 (EAAT5-/-) were recorded in vitro using multielectrode arrays (MEAs). Flicker resolution was considerably lower in EAAT5-/- retinae than in WT retinae. The close proximity to the glutamate release site makes EAAT5 an ideal tool to improve temporal information processing in the retina by controlling information transfer at glutamatergic synapses.
Keywords: EAAT5; glutamate transporter; retina.
Copyright © 2021 Gehlen et al.
Figures
Similar articles
-
Glutamate Transporters EAAT2 and EAAT5 Differentially Shape Synaptic Transmission from Rod Bipolar Cell Terminals.eNeuro. 2022 May 18;9(3):ENEURO.0074-22.2022. doi: 10.1523/ENEURO.0074-22.2022. Print 2022 May-Jun. eNeuro. 2022. PMID: 35523583 Free PMC article.
-
Functional properties of the retinal glutamate transporters GLT-1c and EAAT5.J Biol Chem. 2014 Jan 17;289(3):1815-24. doi: 10.1074/jbc.M113.517177. Epub 2013 Dec 4. J Biol Chem. 2014. PMID: 24307171 Free PMC article.
-
EAAT5 glutamate transporter rapidly binds glutamate with micromolar affinity in mouse rods.J Gen Physiol. 2023 Sep 4;155(9):e202313349. doi: 10.1085/jgp.202313349. Epub 2023 Jul 21. J Gen Physiol. 2023. PMID: 37477643 Free PMC article.
-
EAAT5 Glutamate Transporter-Mediated Inhibition in the Vertebrate Retina.Front Cell Neurosci. 2021 May 6;15:662859. doi: 10.3389/fncel.2021.662859. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34025361 Free PMC article. Review.
-
Cellular Physiology and Pathophysiology of EAAT Anion Channels.Front Cell Neurosci. 2022 Jan 6;15:815279. doi: 10.3389/fncel.2021.815279. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35087380 Free PMC article. Review.
Cited by
-
Synapse Dysfunctions in Multiple Sclerosis.Int J Mol Sci. 2023 Jan 13;24(2):1639. doi: 10.3390/ijms24021639. Int J Mol Sci. 2023. PMID: 36675155 Free PMC article. Review.
-
Glutamate Transporters EAAT2 and EAAT5 Differentially Shape Synaptic Transmission from Rod Bipolar Cell Terminals.eNeuro. 2022 May 18;9(3):ENEURO.0074-22.2022. doi: 10.1523/ENEURO.0074-22.2022. Print 2022 May-Jun. eNeuro. 2022. PMID: 35523583 Free PMC article.
-
Functional maturation of the rod bipolar to AII-amacrine cell ribbon synapse in the mouse retina.Cell Rep. 2023 Nov 28;42(11):113440. doi: 10.1016/j.celrep.2023.113440. Epub 2023 Nov 16. Cell Rep. 2023. PMID: 37976158 Free PMC article.
-
Resolving the molecular architecture of the photoreceptor active zone with 3D-MINFLUX.Sci Adv. 2022 Jul 15;8(28):eabl7560. doi: 10.1126/sciadv.abl7560. Epub 2022 Jul 15. Sci Adv. 2022. PMID: 35857490 Free PMC article.
-
Retinal Glutamate Neurotransmission: From Physiology to Pathophysiological Mechanisms of Retinal Ganglion Cell Degeneration.Life (Basel). 2022 Apr 25;12(5):638. doi: 10.3390/life12050638. Life (Basel). 2022. PMID: 35629305 Free PMC article. Review.
References
-
- Dalet A, Bonsacquet J, Gaboyard-Niay S, Calin-Jageman I, Chidavaenzi RL, Venteo S, Desmadryl G, Goldberg JM, Lysakowski A, Chabbert C (2012) Glutamate transporters EAAT4 and EAAT5 are expressed in vestibular hair cells and calyx endings. PLoS One 7:e46261. 10.1371/journal.pone.0046261 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
