Lipid Catabolism and ROS in Cancer: A Bidirectional Liaison
- PMID: 34771647
- PMCID: PMC8583096
- DOI: 10.3390/cancers13215484
Lipid Catabolism and ROS in Cancer: A Bidirectional Liaison
Abstract
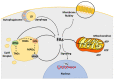
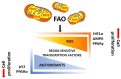
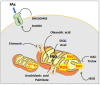
Although cancer cell metabolism was mainly considered to rely on glycolysis, with the concomitant impairment of mitochondrial metabolism, it has recently been demonstrated that several tumor types are sustained by oxidative phosphorylation (OXPHOS). In this context, endogenous fatty acids (FAs) deriving from lipolysis or lipophagy are oxidised into the mitochondrion, and are used as a source of energy through OXPHOS. Because the electron transport chain is the main source of ROS, cancer cells relying on fatty acid oxidation (FAO) need to be equipped with antioxidant systems that maintain the ROS levels under the death threshold. In those conditions, ROS can act as second messengers, favouring proliferation and survival. Herein, we highlight the different responses that tumor cells adopt when lipid catabolism is augmented, taking into account the different ROS fates. Many papers have demonstrated that the pro- or anti-tumoral roles of endogenous FA usage are hugely dependent on the tumor type, and on the capacity of cancer cells to maintain redox homeostasis. In light of this, clinical studies have taken advantage of the boosting of lipid catabolism to increase the efficacy of tumor therapy, whereas, in other contexts, antioxidant compounds are useful to reduce the pro-survival effects of ROS deriving from FAO.
Keywords: fatty acid oxidation; lipid catabolism; mitochondrial metabolism; reactive oxygen species.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
ROS-dependent HIF1α activation under forced lipid catabolism entails glycolysis and mitophagy as mediators of higher proliferation rate in cervical cancer cells.J Exp Clin Cancer Res. 2021 Mar 11;40(1):94. doi: 10.1186/s13046-021-01887-w. J Exp Clin Cancer Res. 2021. PMID: 33706793 Free PMC article.
-
Adaptive antioxidant response to mitochondrial fatty acid oxidation determines the proliferative outcome of cancer cells.Cancer Lett. 2023 Feb 1;554:216010. doi: 10.1016/j.canlet.2022.216010. Epub 2022 Nov 17. Cancer Lett. 2023. PMID: 36402229
-
Genetic and cellular modifiers of oxidative stress: what can we learn from fatty acid oxidation defects?Mol Genet Metab. 2013;110 Suppl:S31-9. doi: 10.1016/j.ymgme.2013.10.007. Epub 2013 Oct 12. Mol Genet Metab. 2013. PMID: 24206932 Review.
-
The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism.Cancer Lett. 2015 Jan 28;356(2 Pt A):156-64. doi: 10.1016/j.canlet.2014.04.001. Epub 2014 Apr 13. Cancer Lett. 2015. PMID: 24732809 Free PMC article. Review.
-
CFTR Deletion Confers Mitochondrial Dysfunction and Disrupts Lipid Homeostasis in Intestinal Epithelial Cells.Nutrients. 2018 Jun 27;10(7):836. doi: 10.3390/nu10070836. Nutrients. 2018. PMID: 29954133 Free PMC article.
Cited by
-
Lipid droplets and polyunsaturated fatty acid trafficking: Balancing life and death.Front Cell Dev Biol. 2023 Jan 27;11:1104725. doi: 10.3389/fcell.2023.1104725. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36776554 Free PMC article. Review.
-
From Crypts to Cancer: A Holistic Perspective on Colorectal Carcinogenesis and Therapeutic Strategies.Int J Mol Sci. 2024 Aug 30;25(17):9463. doi: 10.3390/ijms25179463. Int J Mol Sci. 2024. PMID: 39273409 Free PMC article. Review.
-
Metabolic Reprogramming in Cancer Cells: Emerging Molecular Mechanisms and Novel Therapeutic Approaches.Pharmaceutics. 2022 Jun 19;14(6):1303. doi: 10.3390/pharmaceutics14061303. Pharmaceutics. 2022. PMID: 35745875 Free PMC article. Review.
-
EPHX2 Inhibits Colon Cancer Progression by Promoting Fatty Acid Degradation.Front Oncol. 2022 Mar 30;12:870721. doi: 10.3389/fonc.2022.870721. eCollection 2022. Front Oncol. 2022. PMID: 35433439 Free PMC article.
-
The role of reactive oxygen species in gastric cancer.Cancer Biol Med. 2024 Jul 9;21(9):740-53. doi: 10.20892/j.issn.2095-3941.2024.0182. Cancer Biol Med. 2024. PMID: 38982978 Free PMC article. Review.
References
-
- Skrtić M., Sriskanthadevan S., Jhas B., Gebbia M., Wang X., Wang Z., Hurren R., Jitkova Y., Gronda M., Maclean N., et al. Inhibition of Mitochondrial Translation as a Therapeutic Strategy for Human Acute Myeloid Leukemia. Cancer Cell. 2011;20:674–688. doi: 10.1016/j.ccr.2011.10.015. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

