The Function of the Kynurenine Pathway in the Placenta: A Novel Pharmacotherapeutic Target?
- PMID: 34770059
- PMCID: PMC8582682
- DOI: 10.3390/ijerph182111545
The Function of the Kynurenine Pathway in the Placenta: A Novel Pharmacotherapeutic Target?
Abstract
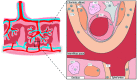
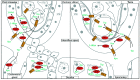
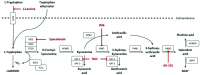
(L-)tryptophan is metabolized via the kynurenine pathway into several kynurenine metabolites with distinct functions. Dysfunction of the kynurenine pathway can lead to impairments in vascular regulation, immune regulation, and tolerance. The first and rate limiting enzyme of this pathway, indoleamine 2,3-dioxygenase (IDO), is highly expressed in the placenta and reduced in placentas from complicated pregnancies. IDO is essential during pregnancy, as IDO inhibition in pregnant mice resulted in fetal loss. However, the exact function of placental IDO, as well as its exact placental localization, remain controversial. This review identified that two isoforms of IDO; IDO1 and IDO2, are differently expressed between placental cells, suggesting spatial segregation. Furthermore, this review summarizes how the placental kynurenine pathway is altered in pregnancy complications, including recurrent miscarriage, preterm birth, preeclampsia, and fetal growth restriction. Importantly, we describe that these alterations do not affect maternally circulating metabolite concentrations, suggesting that the kynurenine pathway functions as a local signaling pathway. In the placenta, it is an important source of de novo placental NAD+ synthesis and regulates fetal tryptophan and kynurenine metabolite supply. Therefore, kynurenine pathway interventions might provide opportunities to treat pregnancy complications, and this review discusses how such treatment could affect placental function and pregnancy development.
Keywords: indoleamine 2,3-dioxygenase; kynurenine; placenta; pregnancy; therapy; tryptophan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
l-Tryptophan-Induced Vasodilation Is Enhanced in Preeclampsia: Studies on Its Uptake and Metabolism in the Human Placenta.Hypertension. 2020 Jul;76(1):184-194. doi: 10.1161/HYPERTENSIONAHA.120.14970. Epub 2020 Jun 1. Hypertension. 2020. PMID: 32475317
-
Localisation of indoleamine 2,3-dioxygenase and kynurenine hydroxylase in the human placenta and decidua: implications for role of the kynurenine pathway in pregnancy.Placenta. 2005 Jul;26(6):498-504. doi: 10.1016/j.placenta.2004.08.009. Placenta. 2005. PMID: 15950064
-
Characterization of the temporal, cell-specific and interferon-inducible patterns of indoleamine 2,3 dioxygenase 1 (IDO1) expression in the human placenta across gestation.Placenta. 2021 Nov;115:129-138. doi: 10.1016/j.placenta.2021.09.008. Epub 2021 Sep 14. Placenta. 2021. PMID: 34619429
-
Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway.Int J Biochem Cell Biol. 2009 Mar;41(3):467-71. doi: 10.1016/j.biocel.2008.01.005. Epub 2008 Jan 11. Int J Biochem Cell Biol. 2009. PMID: 18282734 Review.
-
Indoleamine 2,3-dioxygenase 2 (IDO2) and the kynurenine pathway: characteristics and potential roles in health and disease.Amino Acids. 2013 Dec;45(6):1319-29. doi: 10.1007/s00726-013-1602-1. Epub 2013 Oct 9. Amino Acids. 2013. PMID: 24105077 Review.
Cited by
-
Kynurenines as a Novel Target for the Treatment of Inflammatory Disorders.Cells. 2024 Jul 26;13(15):1259. doi: 10.3390/cells13151259. Cells. 2024. PMID: 39120289 Free PMC article. Review.
-
A Molecular Perspective and Role of NAD+ in Ovarian Aging.Int J Mol Sci. 2024 Apr 25;25(9):4680. doi: 10.3390/ijms25094680. Int J Mol Sci. 2024. PMID: 38731898 Free PMC article. Review.
-
First trimester maternal tryptophan metabolism and embryonic and fetal growth: the Rotterdam Periconceptional Cohort (Predict Study).Hum Reprod. 2024 May 2;39(5):912-922. doi: 10.1093/humrep/deae046. Hum Reprod. 2024. PMID: 38498837 Free PMC article.
-
Metabolomic analysis of the human placenta reveals perturbations in amino acids, purine metabolites, and small organic acids in spontaneous preterm birth.EXCLI J. 2024 Feb 13;23:264-282. doi: 10.17179/excli2023-6785. eCollection 2024. EXCLI J. 2024. PMID: 38487084 Free PMC article.
-
Indoleamine 2,3-Dioxygenase 1 (IDO1) in Kidney Transplantation: A Guardian against Rejection.J Clin Med. 2023 Dec 6;12(24):7531. doi: 10.3390/jcm12247531. J Clin Med. 2023. PMID: 38137602 Free PMC article.
References
-
- Metz R., Duhadaway J.B., Kamasani U., Laury-Kleintop L., Muller A.J., Prendergast G.C. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

