Detection of Nail Oncometabolite SAICAR in Oral Cancer Patients and Its Molecular Interactions with PKM2 Enzyme
- PMID: 34769743
- PMCID: PMC8583651
- DOI: 10.3390/ijerph182111225
Detection of Nail Oncometabolite SAICAR in Oral Cancer Patients and Its Molecular Interactions with PKM2 Enzyme
Abstract
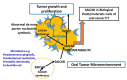
Oncometabolites are known to drive metabolic adaptations in oral cancer. Several oncometabolites are known to be shared between cancer cells and non-cancer cells including microbiotas to modulate the tumor microenvironment. Among potential oncometabolites, succinylaminoimidazolecarboxamide ribose5'-phosphate (SAICAR) supports the growth and invasiveness of cancer cells by pyruvate kinase M2 (PKM2) enzyme in a glucose starved tumor microenvironment. There is a significant gap that shows the detection of SAICAR in biological samples including nails of oral cancer patients. Metabolite identification of SAICAR was investigated in the nails of oral cancer patients using novel vertical tube gel electrophoresis (VTGE) and LC-HRMS. Further molecular docking and molecular dynamics simulations (MDS) were employed to determine the nature of molecular interactions of SAICAR (CHEBI ID:18319) with PKM2 (PDB ID: 4G1N). Molecular docking of SAICAR (CHEBI ID:18319) was performed against pyruvate kinase M2 (PDB ID: 4G1N). Data suggest the presence of oncometabolite SAICAR in nails of oral cancer. Molecular docking of SAICAR with PKM2 showed appreciable binding affinity (-8.0 kcal/mol) with residues including ASP407, THR405, GLU410, ARG443, GLY321, ARG436, HIS439, LYS266, and TYR466. Furthermore, MDS confirmed the specific binding of SAICAR within the activator site of PKM2 and the stability of SAICAR and PKM2 molecular interactions. In conclusion, SAICAR is a promising oncometabolite biomarker present in the nails of oral cancer patients. A significant activation potential of SAICAR exists with the PKM2 enzyme.
Keywords: SAICAR; in silico studies; metabolite biomarkers; oral cancer; pyruvate kinase M2.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



Similar articles
-
SAICAR stimulates pyruvate kinase isoform M2 and promotes cancer cell survival in glucose-limited conditions.Science. 2012 Nov 23;338(6110):1069-72. doi: 10.1126/science.1224409. Epub 2012 Oct 18. Science. 2012. PMID: 23086999 Free PMC article.
-
SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells.Mol Cell. 2014 Mar 6;53(5):700-9. doi: 10.1016/j.molcel.2014.02.015. Mol Cell. 2014. PMID: 24606918 Free PMC article.
-
Succinyl-5-aminoimidazole-4-carboxamide-1-ribose 5'-Phosphate (SAICAR) Activates Pyruvate Kinase Isoform M2 (PKM2) in Its Dimeric Form.Biochemistry. 2016 Aug 23;55(33):4731-6. doi: 10.1021/acs.biochem.6b00658. Epub 2016 Aug 11. Biochemistry. 2016. PMID: 27481063 Free PMC article.
-
Roadmap to Pyruvate Kinase M2 Modulation - A Computational Chronicle.Curr Drug Targets. 2023;24(6):464-483. doi: 10.2174/1389450124666230330103126. Curr Drug Targets. 2023. PMID: 36998144 Review.
-
Pyruvate kinase: Function, regulation and role in cancer.Semin Cell Dev Biol. 2015 Jul;43:43-51. doi: 10.1016/j.semcdb.2015.08.004. Epub 2015 Aug 13. Semin Cell Dev Biol. 2015. PMID: 26277545 Free PMC article. Review.
Cited by
-
Sanguinarine Induces Necroptosis of HCC by Targeting PKM2 Mediated Energy Metabolism.Cancers (Basel). 2024 Jul 13;16(14):2533. doi: 10.3390/cancers16142533. Cancers (Basel). 2024. PMID: 39061173 Free PMC article.
-
Metabolomics and the Multi-Omics View of Cancer.Metabolites. 2022 Feb 7;12(2):154. doi: 10.3390/metabo12020154. Metabolites. 2022. PMID: 35208228 Free PMC article. Review.
References
-
- Farah C.S., Bhatia N., Lalla Y., Vu A., John K., Gupta V., Baeten J., Johnson A., Kademani D. Advances in Early Detection and Diagnostic Adjuncts in Oral Cavity Cancer. In: Kuriakose M.A., editor. Contemporary Oral Oncology: Biology, Epidemiology, Etiology, and Prevention. Springer International Publishing; Cham, Switzerland: 2017. pp. 355–421.
-
- Sankaranarayanan R., Somanathan T., Thomas G., Ramadas K. Screening for Oral Cancer. In: Kuriakose M.A., editor. Contemporary Oral Oncology: Biology, Epidemiology, Etiology, and Prevention. Springer International Publishing; Cham, Switzerland: 2017. pp. 423–444.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

