Focus on the Small GTPase Rab1: A Key Player in the Pathogenesis of Parkinson's Disease
- PMID: 34769517
- PMCID: PMC8584362
- DOI: 10.3390/ijms222112087
Focus on the Small GTPase Rab1: A Key Player in the Pathogenesis of Parkinson's Disease
Abstract
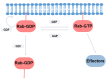
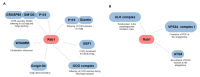
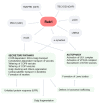
Parkinson's disease (PD) is the second most frequent neurodegenerative disease. It is characterized by the loss of dopaminergic neurons in the substantia nigra and the formation of large aggregates in the survival neurons called Lewy bodies, which mainly contain α-synuclein (α-syn). The cause of cell death is not known but could be due to mitochondrial dysfunction, protein homeostasis failure, and alterations in the secretory/endolysosomal/autophagic pathways. Survival nigral neurons overexpress the small GTPase Rab1. This protein is considered a housekeeping Rab that is necessary to support the secretory pathway, the maintenance of the Golgi complex structure, and the regulation of macroautophagy from yeast to humans. It is also involved in signaling, carcinogenesis, and infection for some pathogens. It has been shown that it is directly linked to the pathogenesis of PD and other neurodegenerative diseases. It has a protective effect against α-σψν toxicity and has recently been shown to be a substrate of LRRK2, which is the most common cause of familial PD and the risk of sporadic disease. In this review, we analyze the key aspects of Rab1 function in dopamine neurons and its implications in PD neurodegeneration/restauration. The results of the current and former research support the notion that this GTPase is a good candidate for therapeutic strategies.
Keywords: GTPases; Golgi fragmentation; LRRK2; Parkinson’s disease; Rab1; autophagy; secretory pathway; α-synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Rab1A over-expression prevents Golgi apparatus fragmentation and partially corrects motor deficits in an alpha-synuclein based rat model of Parkinson's disease.J Parkinsons Dis. 2011;1(4):373-87. doi: 10.3233/JPD-2011-11058. J Parkinsons Dis. 2011. PMID: 23939344
-
Fragmentation of the Golgi complex of dopaminergic neurons in human substantia nigra: New cytopathological findings in Parkinson's disease.Histol Histopathol. 2021 Jan;36(1):47-60. doi: 10.14670/HH-18-270. Epub 2020 Oct 20. Histol Histopathol. 2021. PMID: 33078843
-
LRRK2 and Parkinson's Disease: From Lack of Structure to Gain of Function.Curr Protein Pept Sci. 2017;18(7):677-686. doi: 10.2174/1389203717666160311121748. Curr Protein Pept Sci. 2017. PMID: 26965688 Review.
-
α-Synuclein impairs macroautophagy: implications for Parkinson's disease.J Cell Biol. 2010 Sep 20;190(6):1023-37. doi: 10.1083/jcb.201003122. J Cell Biol. 2010. PMID: 20855506 Free PMC article.
-
LRRK 2 gene mutations in the pathophysiology of the ROCO domain and therapeutic targets for Parkinson's disease: a review.J Biomed Sci. 2018 Jun 14;25(1):52. doi: 10.1186/s12929-018-0454-0. J Biomed Sci. 2018. PMID: 29903014 Free PMC article. Review.
Cited by
-
Host Cell Signatures of the Envelopment Site within Beta-Herpes Virions.Int J Mol Sci. 2022 Sep 1;23(17):9994. doi: 10.3390/ijms23179994. Int J Mol Sci. 2022. PMID: 36077391 Free PMC article. Review.
-
Neurodegenerative Diseases: From Molecular Basis to Therapy.Int J Mol Sci. 2022 Oct 25;23(21):12854. doi: 10.3390/ijms232112854. Int J Mol Sci. 2022. PMID: 36361643 Free PMC article.
-
Deciphering protein prenylation in endocytic trafficking in Toxoplasma gondii.mBio. 2024 Apr 10;15(4):e0028324. doi: 10.1128/mbio.00283-24. Epub 2024 Feb 26. mBio. 2024. PMID: 38407123 Free PMC article.
-
An Update on the Interplay between LRRK2, Rab GTPases and Parkinson's Disease.Biomolecules. 2023 Nov 13;13(11):1645. doi: 10.3390/biom13111645. Biomolecules. 2023. PMID: 38002327 Free PMC article. Review.
-
SIRT3 expression alleviates microglia activation‑induced dopaminergic neuron injury through the mitochondrial pathway.Exp Ther Med. 2022 Sep 7;24(5):662. doi: 10.3892/etm.2022.11598. eCollection 2022 Nov. Exp Ther Med. 2022. PMID: 36168411 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

