Patient-Derived Human Basal and Cutaneous Squamous Cell Carcinoma Tissues Display Apoptosis and Immunomodulation following Gas Plasma Exposure with a Certified Argon Jet
- PMID: 34768877
- PMCID: PMC8584092
- DOI: 10.3390/ijms222111446
Patient-Derived Human Basal and Cutaneous Squamous Cell Carcinoma Tissues Display Apoptosis and Immunomodulation following Gas Plasma Exposure with a Certified Argon Jet
Abstract
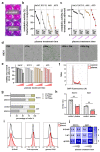
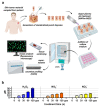
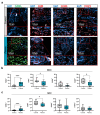
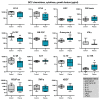
Reactive oxygen species (ROS) have been subject of increasing interest in the pathophysiology and therapy of cancers in recent years. In skin cancer, ROS are involved in UV-induced tumorigenesis and its targeted treatment via, e.g., photodynamic therapy. Another recent technology for topical ROS generation is cold physical plasma, a partially ionized gas expelling dozens of reactive species onto its treatment target. Gas plasma technology is accredited for its wound-healing abilities in Europe, and current clinical evidence suggests that it may have beneficial effects against actinic keratosis. Since the concept of hormesis dictates that low ROS levels perform signaling functions, while high ROS levels cause damage, we investigated herein the antitumor activity of gas plasma in non-melanoma skin cancer. In vitro, gas plasma exposure diminished the metabolic activity, preferentially in squamous cell carcinoma cell (SCC) lines compared to non-malignant HaCaT cells. In patient-derived basal cell carcinoma (BCC) and SCC samples treated with gas plasma ex vivo, increased apoptosis was found in both cancer types. Moreover, the immunomodulatory actions of gas plasma treatment were found affecting, e.g., the expression of CD86 and the number of regulatory T-cells. The supernatants of these ex vivo cultured tumors were quantitatively screened for cytokines, chemokines, and growth factors, identifying CCL5 and GM-CSF, molecules associated with skin cancer metastasis, to be markedly decreased. These findings suggest gas plasma treatment to be an interesting future technology for non-melanoma skin cancer topical therapy.
Keywords: ROS; chemokines; cold physical plasma; cytokines; reactive oxygen species; skin cancer.
Conflict of interest statement
The authors report no conflict of interest.
Figures

Similar articles
-
Optimization of the Treatment of Squamous Cell Carcinoma Cells by Combining Photodynamic Therapy with Cold Atmospheric Plasma.Int J Mol Sci. 2024 Oct 8;25(19):10808. doi: 10.3390/ijms251910808. Int J Mol Sci. 2024. PMID: 39409136 Free PMC article.
-
Lamin expression in normal human skin, actinic keratosis, squamous cell carcinoma and basal cell carcinoma.Br J Dermatol. 2003 Jan;148(1):102-9. doi: 10.1046/j.1365-2133.2003.05026.x. Br J Dermatol. 2003. PMID: 12534602
-
Thermal photodynamic therapy increases apoptosis and reactive oxygen species generation in cutaneous and mucosal squamous cell carcinoma cells.Sci Rep. 2018 Aug 22;8(1):12599. doi: 10.1038/s41598-018-30908-6. Sci Rep. 2018. PMID: 30135507 Free PMC article.
-
UV-Induced Molecular Signaling Differences in Melanoma and Non-melanoma Skin Cancer.Adv Exp Med Biol. 2017;996:27-40. doi: 10.1007/978-3-319-56017-5_3. Adv Exp Med Biol. 2017. PMID: 29124688 Review.
-
Ultraviolet damage, DNA repair and vitamin D in nonmelanoma skin cancer and in malignant melanoma: an update.Adv Exp Med Biol. 2014;810:208-33. doi: 10.1007/978-1-4939-0437-2_12. Adv Exp Med Biol. 2014. PMID: 25207368 Review.
Cited by
-
Plasma Biology 2.0.Int J Mol Sci. 2022 Mar 28;23(7):3684. doi: 10.3390/ijms23073684. Int J Mol Sci. 2022. PMID: 35409044 Free PMC article.
-
Cold atmospheric plasma (CAP): a revolutionary approach in dermatology and skincare.Eur J Med Res. 2024 Oct 5;29(1):487. doi: 10.1186/s40001-024-02088-9. Eur J Med Res. 2024. PMID: 39367460 Free PMC article. Review.
-
Medical gas plasma technology: Roadmap on cancer treatment and immunotherapy.Redox Biol. 2023 Sep;65:102798. doi: 10.1016/j.redox.2023.102798. Epub 2023 Jun 27. Redox Biol. 2023. PMID: 37556976 Free PMC article. Review.
-
Oxidized Melanoma Antigens Promote Activation and Proliferation of Cytotoxic T-Cell Subpopulations.Adv Sci (Weinh). 2024 Sep;11(33):e2404131. doi: 10.1002/advs.202404131. Epub 2024 Jul 3. Adv Sci (Weinh). 2024. PMID: 38958560 Free PMC article.
-
Gas Plasma Exposure of Glioblastoma Is Cytotoxic and Immunomodulatory in Patient-Derived GBM Tissue.Cancers (Basel). 2022 Feb 5;14(3):813. doi: 10.3390/cancers14030813. Cancers (Basel). 2022. PMID: 35159079 Free PMC article.
References
-
- Georgescu S.R., Mitran C.I., Mitran M.I., Caruntu C., Caruntu A., Lupu M., Matei C., Constantin C., Neagu M. Tumour microenvironment in skin carcinogenesis. Tumor Microenviron. Organs. 2020;1226:123–142. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

