The pathophysiology of leg cramping during dialysis and the use of carnitine in its treatment
- PMID: 34762357
- PMCID: PMC8582296
- DOI: 10.14814/phy2.15114
The pathophysiology of leg cramping during dialysis and the use of carnitine in its treatment
Abstract
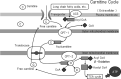
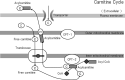
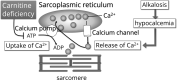
Leg cramping is a common side effect of hemodialysis, and this is frequently treated by the administration of carnitine, but this is not effective in every patient. Alkalosis is a key component of the etiology of leg cramping during hemodialysis sessions. This is mediated through the binding of calcium ions to serum albumin, which causes hypocalcemia, and an increase in the release of calcium ions from the sarcoplasmic reticulum. Normally the calcium pump on the sarcoplasmic reticulum consumes ATP and quickly reuptakes the released calcium ions, which rapidly stops excessive muscle contractions. Thus, carnitine deficiency results in prolonged muscle contraction because of ATP depletion. However, during ATP production, carnitine is only involved up to the stage of acyl-CoA transport into mitochondria, and for the efficient generation of ATP, the subsequent metabolism of acyl-CoA is also important. For example, β-oxidation and the tricarboxylic acid cycle may be affected by a deficiency of water-soluble vitamins and the electron transport chain requires coenzyme Q10, but statins inhibit its production. The resulting accumulation of excess long-chain acyl-CoA in mitochondria inhibits enzymes involved in energy production. Thus, carnitine administration may be used more effectively if clinicians are aware of its specific physiologic roles.
Keywords: ATP; acyl coenzyme A; carnitine; coenzyme Q10; contraction alkalosis; leg cramping; muscle cramp.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Effects of L-carnitine on dialysis-related hypotension and muscle cramps: a meta-analysis.Am J Kidney Dis. 2008 Nov;52(5):962-71. doi: 10.1053/j.ajkd.2008.05.031. Epub 2008 Aug 15. Am J Kidney Dis. 2008. PMID: 18706751 Review.
-
Aspects of long-chain acyl-COA metabolism.Mol Cell Biochem. 1975 Apr 30;7(1):19-31. doi: 10.1007/BF01732160. Mol Cell Biochem. 1975. PMID: 1134497
-
Correlation between increased serum and tissue L-carnitine levels and improved muscle symptoms in hemodialyzed patients.Am J Clin Nutr. 1983 Oct;38(4):523-31. doi: 10.1093/ajcn/38.4.523. Am J Clin Nutr. 1983. PMID: 6624694 Clinical Trial.
-
Can L-carnitine supplementation and exercise improve muscle complications in patients with liver cirrhosis who receive branched-chain amino acid supplementation?Eur J Gastroenterol Hepatol. 2019 Jul;31(7):878-884. doi: 10.1097/MEG.0000000000001368. Eur J Gastroenterol Hepatol. 2019. PMID: 31150367
-
Muscle cramps during hemodialysis.Int J Artif Organs. 1994 Nov;17(11):570-2. Int J Artif Organs. 1994. PMID: 7744514 Review. No abstract available.
Cited by
-
Zinc Supplementation Enhances the Hematopoietic Activity of Erythropoiesis-Stimulating Agents but Not Hypoxia-Inducible Factor-Prolyl Hydroxylase Inhibitors.Nutrients. 2024 Feb 13;16(4):520. doi: 10.3390/nu16040520. Nutrients. 2024. PMID: 38398842 Free PMC article.
-
Epidemiology of peritoneal dialysis outcomes.Nat Rev Nephrol. 2022 Dec;18(12):779-793. doi: 10.1038/s41581-022-00623-7. Epub 2022 Sep 16. Nat Rev Nephrol. 2022. PMID: 36114414 Free PMC article. Review.
-
Nocturnal Leg Cramping Caused by Carnitine Deficiency Due to Long-Term Pivalate Antibiotics Administration in a Patient With Chronic Kidney Disease.Cureus. 2023 Nov 16;15(11):e48927. doi: 10.7759/cureus.48927. eCollection 2023 Nov. Cureus. 2023. PMID: 38106710 Free PMC article.
-
Factors Affecting Pain in Hemodialysis and Non-pharmacological Management.Cureus. 2023 Feb 25;15(2):e35448. doi: 10.7759/cureus.35448. eCollection 2023 Feb. Cureus. 2023. PMID: 36994274 Free PMC article. Review.
-
Prevention of Intradialytic Hypotension in Hemodialysis Patients: Current Challenges and Future Prospects.Int J Nephrol Renovasc Dis. 2023 Aug 1;16:173-181. doi: 10.2147/IJNRD.S245621. eCollection 2023. Int J Nephrol Renovasc Dis. 2023. PMID: 37547077 Free PMC article. Review.
References
-
- Beca, S. , Aschar‐Sobbi, R. , Ponjevic, D. , Winkfein, R. J. , Kargacin, M. E. , & Kargacin, G. J. (2009). Effects of monovalent cations on Ca2+ uptake by skeletal and cardiac muscle sarcoplasmic reticulum. Archives of Biochemistry and Biophysics, 490(2), 110–117. 10.1016/j.abb.2009.08.014 - DOI - PubMed
-
- Bennett, A. L. , Chao, C. C. , Hu, S. , Buchwald, D. , Fagioli, L. R. , Schur, P. H. , Peterson, P. K. , & Komaroff, A. L. (1997). Elevation of bioactive transforming growth factor‐beta in serum from patients with chronic fatigue syndrome. Journal of Clinical Immunology, 17(2), 160–166. 10.1023/a:1027330616073 - DOI - PubMed
-
- Borum, P. R. , & Taggart, E. M. (1986). Carnitine nutriture of dialysis patients. Journal of the American Dietetic Association, 86(5), 644–647. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

