Umbelliferyloxymethyl phosphonate compounds-weakly binding zinc ionophores with neuroprotective properties
- PMID: 34761777
- PMCID: PMC8631114
- DOI: 10.1039/d1dt02298a
Umbelliferyloxymethyl phosphonate compounds-weakly binding zinc ionophores with neuroprotective properties
Abstract
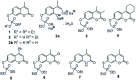
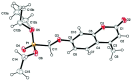
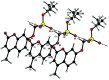
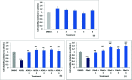
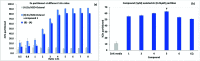
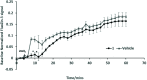
Umbelliferone is a member of the coumarin family of compounds which are known for diverse pharmacological activity including in targets relevant to Alzheimers disease, AD. The toxicity associated with some forms of the amyloid protein, Aβ, and the role of Zn2+ (and other biometals) dyshomeostasis in this, are of great interest in AD and make metal ionophore capability desirable in so called multi target drug ligands MTDLs. A new series of umbelliferyloxymethyl phosphonic acid diethylester compounds (umbelliferyloxymethyl phosphonates) bearing a phosphonate at the 7-position (compounds 1, 3-6), hydrolysis products 2, 2a and 2b from 1 and analogues 7 and 8 of 1 with 7-O to 7-S and 1-O to 1-NH substitutions, are reported. Single crystal X-ray structures of compounds 1, 2 and 2a were determined. In terms of neuroprotective properties, the compounds 1, 2, 3, 4, 5 and 6 at 1 μM concentration, inhibited the toxicity of Aβ1-42 (Aβ42) in both toxic Amyloid Derived Diffusible Ligand (ADDL) and fibrillar (fibril) forms towards rat hippocampal cells. Compound 7 displayed cytotoxicity and 8 failed to inhibit Aβ42 toxicity. Concerning compound-metal ionophore activity (assessed using chemical experiments), despite weak binding to Zn2+ determined from 31P NMR titration of 1 and 2 by ZnCl2, compounds 1, 3, 4, 5 and 6 demonstrated ionophore assisted partition of Zn2+ from water to octanol at micromolar concentrations with efficacy on a par with or better than the chelator MTDL clioquinol (5-chloro-7-iodo-8-hydroxyquinoline). Partition was assessed using furnace Atomic Absorption Spectroscopy (AAS). In further experiments interaction of compound 1 with Zn2+ or it's pathways was inferred by (i) delayed fluorescence response with added Zn2+ in cells treated with FluoZin-3 and (ii) by suppression of Zn2+ promoted aggregation of Aβ42.
Conflict of interest statement
There are no conflicts to declare.
Figures
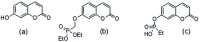
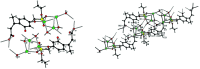

Similar articles
-
Multi-target-directed triazole derivatives as promising agents for the treatment of Alzheimer's disease.Bioorg Chem. 2019 Jun;87:572-584. doi: 10.1016/j.bioorg.2019.03.058. Epub 2019 Mar 23. Bioorg Chem. 2019. PMID: 30928879
-
Identification of a Novel Multifunctional Ligand for Simultaneous Inhibition of Amyloid-Beta (Aβ42) and Chelation of Zinc Metal Ion.ACS Chem Neurosci. 2019 Nov 20;10(11):4619-4632. doi: 10.1021/acschemneuro.9b00468. Epub 2019 Oct 10. ACS Chem Neurosci. 2019. PMID: 31566950
-
Protection against amyloid beta peptide toxicity by zinc.Brain Res. 1999 Mar 27;823(1-2):88-95. doi: 10.1016/s0006-8993(99)01114-2. Brain Res. 1999. PMID: 10095015
-
Sarsasapogenin: A steroidal saponin from Asparagus racemosus as multi target directed ligand in Alzheimer's disease.Steroids. 2020 Jan;153:108529. doi: 10.1016/j.steroids.2019.108529. Epub 2019 Oct 28. Steroids. 2020. PMID: 31672628
-
Mechanistic Insight into the Design of Chemical Tools to Control Multiple Pathogenic Features in Alzheimer's Disease.Acc Chem Res. 2021 Oct 19;54(20):3930-3940. doi: 10.1021/acs.accounts.1c00457. Epub 2021 Oct 4. Acc Chem Res. 2021. PMID: 34606227 Review.
Cited by
-
7,8-Dihydroxycoumarin Alleviates Synaptic Loss by Activated PI3K-Akt-CREB-BDNF Signaling in Alzheimer's Disease Model Mice.J Agric Food Chem. 2022 Jun 15;70(23):7130-7138. doi: 10.1021/acs.jafc.2c02140. Epub 2022 Jun 3. J Agric Food Chem. 2022. PMID: 35657168 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

