Undisclosed payments by pharmaceutical manufacturers to authors of inflammatory bowel disease guidelines in the United States
- PMID: 34755712
- PMCID: PMC8656332
- DOI: 10.4103/sjg.sjg_426_21
Undisclosed payments by pharmaceutical manufacturers to authors of inflammatory bowel disease guidelines in the United States
Abstract
Background: Payments from pharmaceutical drug manufacturers to authors of clinical practice guidelines (CPGs) may have an impact on their recommendations. In this study, we aimed to evaluate the accuracy of financial conflict of interest (FCOI) declarations among authors of Inflammatory Bowel Disease (IBD) guidelines.
Methods: We collected data on industry payments to authors of IBD guidelines published by the American Gastroenterology Association (AGA), American College of Gastroenterology (ACG) and American Society of Gastrointestinal Endoscopy (ASGE). We reported the accuracy of the authors' declarations by comparing their statements in the FCOI section of the guidelines with the data reported on the Centers for Medicare and Medicaid Services website (CMS-OP). We also investigated the adherence of IBD guidelines to the National Academy of Medicine (NAM) criteria for trustworthy guidelines.
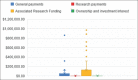
Results: A total of eight clinical practice guidelines and 35 individual authors were included. Four authors had no profile identified at CMS-OP. The total payment to all included authors was $10,575,843.06, with a mean payment of $314,242.38 per author. A total of 28/35 authors (80%) received payment from pharmaceutical companies, 23/35 (65.7%) received $10,000 or more, 15/35 (42.8%) received $100,000 or more and 3/35 (8.57%) received $1,000,000 or more. Total discrepancies identified while comparing the authors' declaration of their FCOI and CMS-OP were 28: ACG had 12/14 (85.7%), AGA had 7/12 (53.8%) and ASGE had 9/10 (90%) discrepancies. None of the guidelines met all NAM criteria and 4/8 (50%) guidelines met none.
Conclusions: Discrepancies exist between authors' declarations in the FOCI section and data on CMS-OP. Poor compliance with the NAM criteria was prevalent among authors of IBD guidelines. More transparency in reporting and monitoring is needed.
Keywords: Conflict of interest; financial declaration; guidelines; inflammatory bowel disease.
Conflict of interest statement
None
Figures
Comment in
-
Despise the free lunch.Saudi J Gastroenterol. 2021 Nov-Dec;27(6):317-318. doi: 10.4103/sjg.sjg_541_21. Saudi J Gastroenterol. 2021. PMID: 34755713 Free PMC article. No abstract available.
Similar articles
-
Undisclosed payments by pharmaceutical and medical device manufacturers to authors of endoscopy guidelines in the United States.Gastrointest Endosc. 2020 Feb;91(2):266-273. doi: 10.1016/j.gie.2019.11.010. Epub 2019 Nov 15. Gastrointest Endosc. 2020. PMID: 31738925
-
Financial Conflicts of Interest in Inflammatory Bowel Disease Guidelines.Inflamm Bowel Dis. 2019 Mar 14;25(4):642-645. doi: 10.1093/ibd/izy315. Inflamm Bowel Dis. 2019. PMID: 30295831
-
Evaluation of Pharmaceutical Company Payments and Conflict of Interest Disclosures Among Oncology Clinical Practice Guideline Authors in Japan.JAMA Netw Open. 2019 Apr 5;2(4):e192834. doi: 10.1001/jamanetworkopen.2019.2834. JAMA Netw Open. 2019. PMID: 31026027 Free PMC article.
-
Financial Conflicts of Interest in Clinical Practice Guidelines: A Systematic Review.Mayo Clin Proc Innov Qual Outcomes. 2021 Jan 19;5(2):466-475. doi: 10.1016/j.mayocpiqo.2020.09.016. eCollection 2021 Apr. Mayo Clin Proc Innov Qual Outcomes. 2021. PMID: 33997642 Free PMC article. Review.
-
The undisclosed disclosures: The dollar-outcome relationship in resuscitative endovascular balloon occlusion of the aorta.J Trauma Acute Care Surg. 2023 Nov 1;95(5):726-730. doi: 10.1097/TA.0000000000004080. Epub 2023 Jun 15. J Trauma Acute Care Surg. 2023. PMID: 37316993 Review.
Cited by
-
Despise the free lunch.Saudi J Gastroenterol. 2021 Nov-Dec;27(6):317-318. doi: 10.4103/sjg.sjg_541_21. Saudi J Gastroenterol. 2021. PMID: 34755713 Free PMC article. No abstract available.
-
Nonalcoholic Steatohepatitis, Peroxisome Proliferator-Activated Receptors and Our Good Glitazar: Proof of the Pudding is in the Eating.J Clin Exp Hepatol. 2022 Mar-Apr;12(2):263-267. doi: 10.1016/j.jceh.2022.01.008. Epub 2022 Jan 25. J Clin Exp Hepatol. 2022. PMID: 35535098 Free PMC article. No abstract available.
References
-
- Institute of Medicine, Board on Health Care Services, Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011.
-
- Lo B, Field MJ, editors. Institute of Medicine (US) Committee on Conflict of Interest in Medical Research, Education, and Practice. Conflict of Interest in Medical Research, Education, and Practice. Washington, DC: National Academies Press (US); 2010. - PubMed
-
- The Patient Protection and Affordable Care Act. Washington, DC: U.S. Government Publishing Office; 2010.
-
- Institute of Medicine (US). Committee on Standards for Developing Trustworthy Clinical Practice Guidelines. Clinical Practice Guidelines We Can Trust. Washington, DC: National Academies Press; 2011. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous