Bunyavirus SFTSV exploits autophagic flux for viral assembly and egress
- PMID: 34747299
- PMCID: PMC9298452
- DOI: 10.1080/15548627.2021.1994296
Bunyavirus SFTSV exploits autophagic flux for viral assembly and egress
Abstract
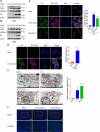
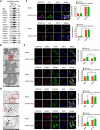
Severe fever with thrombocytopenia syndrome virus (SFTSV) is an emerging negatively stranded enveloped RNA bunyavirus that causes SFTS with a high case fatality rate of up to 30%. Macroautophagy/autophagy is an evolutionarily conserved process involved in the maintenance of host homeostasis, which exhibits anti-viral or pro-viral responses in reaction to different viral challenges. However, the interaction between the bunyavirus SFTSV and the autophagic process is still largely unclear. By establishing various autophagy-deficient cell lines, we found that SFTSV triggered RB1CC1/FIP200-BECN1-ATG5-dependent classical autophagy flux. SFTSV nucleoprotein induced BECN1-dependent autophagy by disrupting the BECN1-BCL2 association. Importantly, SFTSV utilized autophagy for the viral life cycle, which not only assembled in autophagosomes derived from the ERGIC and Golgi complex, but also utilized autophagic vesicles for exocytosis. Taken together, our results suggest a novel virus-autophagy interaction model in which bunyavirus SFTSV induces classical autophagy flux for viral assembly and egress processes, suggesting that autophagy inhibition may be a novel therapy for treating or releasing SFTS.
Keywords: Autophagy; bunyavirus; sftsv; viral assembly; viral egress.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
SFTSV nucleoprotein mediates DNA sensor cGAS degradation to suppress cGAS-dependent antiviral responses.Microbiol Spectr. 2024 Jun 4;12(6):e0379623. doi: 10.1128/spectrum.03796-23. Epub 2024 May 7. Microbiol Spectr. 2024. PMID: 38712963 Free PMC article.
-
The SFTSV Nonstructural Proteins Induce Autophagy to Promote Viral Replication via Interaction with Vimentin.J Virol. 2023 Apr 27;97(4):e0030223. doi: 10.1128/jvi.00302-23. Epub 2023 Apr 11. J Virol. 2023. PMID: 37039677 Free PMC article.
-
SFTS bunyavirus NSs protein sequestrates mTOR into inclusion bodies and deregulates mTOR-ULK1 signaling, provoking pro-viral autophagy.J Med Virol. 2023 Jan;95(1):e28371. doi: 10.1002/jmv.28371. J Med Virol. 2023. PMID: 36458534
-
Severe fever with thrombocytopenia syndrome virus: a highly lethal bunyavirus.Crit Rev Microbiol. 2021 Feb;47(1):112-125. doi: 10.1080/1040841X.2020.1847037. Epub 2020 Nov 27. Crit Rev Microbiol. 2021. PMID: 33245676 Review.
-
Severe fever with thrombocytopenia syndrome and its pathogen SFTSV.Microbes Infect. 2015 Feb;17(2):149-54. doi: 10.1016/j.micinf.2014.12.002. Epub 2014 Dec 11. Microbes Infect. 2015. PMID: 25498868 Review.
Cited by
-
Taurolithocholic acid protects against viral haemorrhagic fever via inhibition of ferroptosis.Nat Microbiol. 2024 Oct;9(10):2583-2599. doi: 10.1038/s41564-024-01801-y. Epub 2024 Sep 18. Nat Microbiol. 2024. PMID: 39294459
-
Recent Advances in the Study of the Immune Escape Mechanism of SFTSV and Its Therapeutic Agents.Viruses. 2023 Apr 10;15(4):940. doi: 10.3390/v15040940. Viruses. 2023. PMID: 37112920 Free PMC article. Review.
-
Rift Valley Fever Virus Nucleoprotein Triggers Autophagy to Dampen Antiviral Innate Immune Responses.J Virol. 2023 Apr 27;97(4):e0181422. doi: 10.1128/jvi.01814-22. Epub 2023 Mar 20. J Virol. 2023. PMID: 36939341 Free PMC article.
-
Pathogenesis and virulence of Heartland virus.Virulence. 2024 Dec;15(1):2348252. doi: 10.1080/21505594.2024.2348252. Epub 2024 May 7. Virulence. 2024. PMID: 38712703 Free PMC article. Review.
-
Five questions on the cell-to-cell movement of Orthotospoviruses.BBA Adv. 2024 Oct 16;6:100124. doi: 10.1016/j.bbadva.2024.100124. eCollection 2024. BBA Adv. 2024. PMID: 39498475 Free PMC article.
References
-
- Li H, Lu QB, Xing B, et al. Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011-17: a prospective observational study. Lancet Infect Dis. 2018;18:1127–1137. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
