Autophagy Modulation by Viral Infections Influences Tumor Development
- PMID: 34745965
- PMCID: PMC8569469
- DOI: 10.3389/fonc.2021.743780
Autophagy Modulation by Viral Infections Influences Tumor Development
Abstract
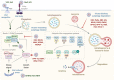
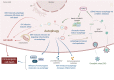
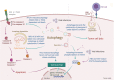
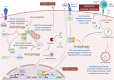
Autophagy is a self-degradative process important for balancing cellular homeostasis at critical times in development and/or in response to nutrient stress. This is particularly relevant in tumor model in which autophagy has been demonstrated to have an important impact on tumor behavior. In one hand, autophagy limits tumor transformation of precancerous cells in early stage, and in the other hand, it favors the survival, proliferation, metastasis, and resistance to antitumor therapies in more advanced tumors. This catabolic machinery can be induced by an important variety of extra- and intracellular stimuli. For instance, viral infection has often been associated to autophagic modulation, and the role of autophagy in virus replication differs according to the virus studied. In the context of tumor development, virus-modulated autophagy can have an important impact on tumor cells' fate. Extensive analyses have shed light on the molecular and/or functional complex mechanisms by which virus-modulated autophagy influences precancerous or tumor cell development. This review includes an overview of discoveries describing the repercussions of an autophagy perturbation during viral infections on tumor behavior.
Keywords: autophagy; immunity; oncogenic virus; oncolytic virus; tumor development and progression; tumor resistance; tumorigenesis; viral infection.
Copyright © 2021 Leonardi, Sibéril, Alifano, Cremer and Joubert.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
[Autophagy modulation by viruses: An important role in tumor progression].Med Sci (Paris). 2022 Feb;38(2):159-167. doi: 10.1051/medsci/2022010. Epub 2022 Feb 18. Med Sci (Paris). 2022. PMID: 35179470 Review. French.
-
Autophagy in Viral Development and Progression of Cancer.Front Oncol. 2021 Mar 8;11:603224. doi: 10.3389/fonc.2021.603224. eCollection 2021. Front Oncol. 2021. PMID: 33763351 Free PMC article. Review.
-
Oncolytic Virus-Induced Autophagy in Glioblastoma.Cancers (Basel). 2021 Jul 12;13(14):3482. doi: 10.3390/cancers13143482. Cancers (Basel). 2021. PMID: 34298694 Free PMC article. Review.
-
The Interplay of HIV and Autophagy in Early Infection.Front Microbiol. 2021 Apr 28;12:661446. doi: 10.3389/fmicb.2021.661446. eCollection 2021. Front Microbiol. 2021. PMID: 33995324 Free PMC article. Review.
-
Crosstalk between oncolytic viruses and autophagy in cancer therapy.Biomed Pharmacother. 2021 Feb;134:110932. doi: 10.1016/j.biopha.2020.110932. Epub 2020 Dec 25. Biomed Pharmacother. 2021. PMID: 33370632 Review.
Cited by
-
Viruses Binding to Host Receptors Interacts with Autophagy.Int J Mol Sci. 2023 Feb 8;24(4):3423. doi: 10.3390/ijms24043423. Int J Mol Sci. 2023. PMID: 36834833 Free PMC article. Review.
-
The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities.Cell Prolif. 2022 Sep;55(9):e13275. doi: 10.1111/cpr.13275. Epub 2022 Jun 26. Cell Prolif. 2022. PMID: 35754255 Free PMC article. Review.
-
Mechanisms of Survival of Cytomegalovirus-Infected Tumor Cells.Mol Biol. 2022;56(5):668-683. doi: 10.1134/S0026893322050132. Epub 2022 Oct 5. Mol Biol. 2022. PMID: 36217337 Free PMC article.
-
Curcumin ameliorates H2O2-induced inflammatory response in chondrocytes by inducing autophagy activation.Exp Ther Med. 2022 Apr;23(4):272. doi: 10.3892/etm.2022.11198. Epub 2022 Feb 9. Exp Ther Med. 2022. PMID: 35251338 Free PMC article.
-
Host immune responses against African swine fever virus: Insights and challenges for vaccine development.Open Vet J. 2023 Dec;13(12):1517-1535. doi: 10.5455/OVJ.2023.v13.i12.2. Epub 2023 Dec 31. Open Vet J. 2023. PMID: 38292721 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources

