Colony stimulating factors in the nervous system
- PMID: 34743926
- PMCID: PMC8671346
- DOI: 10.1016/j.smim.2021.101511
Colony stimulating factors in the nervous system
Abstract
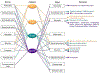
Although traditionally seen as regulators of hematopoiesis, colony-stimulating factors (CSFs) have emerged as important players in the nervous system, both in health and disease. This review summarizes the cellular sources, patterns of expression and physiological roles of the macrophage (CSF-1, IL-34), granulocyte-macrophage (GM-CSF) and granulocyte (G-CSF) colony stimulating factors within the nervous system, with a particular focus on their actions on microglia. CSF-1 and IL-34, via the CSF-1R, are required for the development, proliferation and maintenance of essentially all CNS microglia in a temporal and regional specific manner. In contrast, in steady state, GM-CSF and G-CSF are mainly involved in regulation of microglial function. The alterations in expression of these growth factors and their receptors, that have been reported in several neurological diseases, are described and the outcomes of their therapeutic targeting in mouse models and humans are discussed.
Keywords: CSF-1; CSF-1R; G-CSF; GM-CSF; IL-34; Microglia.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Figures
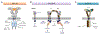
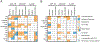
Similar articles
-
Regulation of myeloid development and function by colony stimulating factors.Dev Comp Immunol. 2004 May 3;28(5):509-54. doi: 10.1016/j.dci.2003.09.010. Dev Comp Immunol. 2004. PMID: 15062647 Review.
-
Colony-stimulating factor 1 receptor signaling in the central nervous system and the potential of its pharmacological inhibitors to halt the progression of neurological disorders.Inflammopharmacology. 2022 Jun;30(3):821-842. doi: 10.1007/s10787-022-00958-4. Epub 2022 Mar 15. Inflammopharmacology. 2022. PMID: 35290551 Review.
-
Effects of recombinant human granulocyte colony-stimulating factor (CSF), human granulocyte macrophage-CSF, and gibbon interleukin-3 on hematopoiesis in human long-term bone marrow culture.Blood. 1990 Jun 1;75(11):2118-29. Blood. 1990. PMID: 1693295
-
Mechanism of synergy between granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in colony formation from human marrow cells in vitro.Exp Hematol. 1989 Aug;17(7):816-21. Exp Hematol. 1989. PMID: 2473914
-
Interleukin-1, tumor necrosis factor, and the production of colony-stimulating factors by cultured mesenchymal cells.Blood. 1988 Oct;72(4):1316-23. Blood. 1988. PMID: 2458780
Cited by
-
Inflammatory checkpoints in amyotrophic lateral sclerosis: From biomarkers to therapeutic targets.Front Immunol. 2022 Dec 22;13:1059994. doi: 10.3389/fimmu.2022.1059994. eCollection 2022. Front Immunol. 2022. PMID: 36618399 Free PMC article.
-
Mammalian Animal and Human Retinal Organ Culture as Pre-Clinical Model to Evaluate Oxidative Stress and Antioxidant Intraocular Therapeutics.Antioxidants (Basel). 2023 Jun 3;12(6):1211. doi: 10.3390/antiox12061211. Antioxidants (Basel). 2023. PMID: 37371942 Free PMC article.
-
Modulation of central synapse remodeling after remote peripheral injuries by the CCL2-CCR2 axis and microglia.Cell Rep. 2024 Feb 27;43(2):113776. doi: 10.1016/j.celrep.2024.113776. Epub 2024 Feb 15. Cell Rep. 2024. PMID: 38367237 Free PMC article.
-
New Insights into Microglial Mechanisms of Memory Impairment in Alzheimer's Disease.Biomolecules. 2022 Nov 21;12(11):1722. doi: 10.3390/biom12111722. Biomolecules. 2022. PMID: 36421736 Free PMC article. Review.
-
Elevated granulocyte colony stimulating factor (CSF) causes cerebellar deficits and anxiety in a model of CSF-1 receptor related leukodystrophy.Glia. 2023 Mar;71(3):775-794. doi: 10.1002/glia.24310. Epub 2022 Nov 26. Glia. 2023. PMID: 36433736 Free PMC article.
References
-
- Chitu V, Caescu CI, Stanley ER, Lennartsson J, Ronnstrand L, Heldin C-H, PDGF Receptor Family, in: Wheeler DL, Yarden Y (Eds.), The Receptor Tyrosine Kinases: Family and Subfamilies, Springer Science, New York, 2015, pp. 373–538.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

