Precise Diabetic Wound Therapy: PLS Nanospheres Eliminate Senescent Cells via DPP4 Targeting and PARP1 Activation
- PMID: 34738744
- PMCID: PMC8728814
- DOI: 10.1002/advs.202104128
Precise Diabetic Wound Therapy: PLS Nanospheres Eliminate Senescent Cells via DPP4 Targeting and PARP1 Activation
Abstract
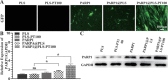
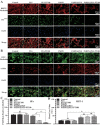
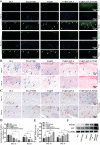
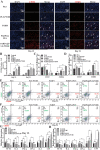
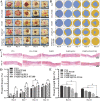
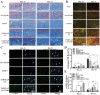
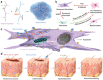
Diabetic ulcers, a difficult problem faced by clinicians, are strongly associated with an increase in cellular senescence. Few empirical studies have focused on exploring a targeted strategy to cure diabetic wounds by eliminating senescent fibroblasts (SFs) and reducing side effects. In this study, poly-l-lysine/sodium alginate (PLS) is modified with talabostat (PT100) and encapsulates a PARP1 plasmid (PARP1@PLS-PT100) for delivery to target the dipeptidyl peptidase 4 (DPP4) receptor and eliminate SFs. PARP1@PLS-PT100 releases encapsulated plasmids, displaying high selectivity for SFs over normal fibroblasts by targeting the DPP4 receptor, decreasing senescence-associated secretory phenotypes (SASPs), and stimulating the secretion of anti-inflammatory factors. Furthermore, the increased apoptosis of SFs and the disappearance of cellular senescence alleviates SASPs, accelerates re-epithelialization and collagen deposition, and significantly induces macrophage M2 polarization, which mediates tissue repair and the inflammatory response. This innovative strategy has revealed the previously undefined role of PARP1@PLS-PT100 in promoting diabetic wound healing, suggesting its therapeutic potential in refractory wound repair.
Keywords: DPP4 receptor; diabetic wound healing; nanospheres; selective targeting; senescence.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Enzyme-responsive nanospheres target senescent cells for diabetic wound healing by employing chemodynamic therapy.Acta Biomater. 2023 Dec;172:407-422. doi: 10.1016/j.actbio.2023.10.015. Epub 2023 Oct 15. Acta Biomater. 2023. PMID: 37848101
-
Dipeptidyl peptidase-4 inhibition improves endothelial senescence by activating AMPK/SIRT1/Nrf2 signaling pathway.Biochem Pharmacol. 2020 Jul;177:113951. doi: 10.1016/j.bcp.2020.113951. Epub 2020 Apr 3. Biochem Pharmacol. 2020. PMID: 32251672
-
Elevated Local Senescence in Diabetic Wound Healing Is Linked to Pathological Repair via CXCR2.J Invest Dermatol. 2019 May;139(5):1171-1181.e6. doi: 10.1016/j.jid.2019.01.005. Epub 2019 Jan 23. J Invest Dermatol. 2019. PMID: 30684552
-
PARP1 at the crossroad of cellular senescence and nucleolar processes.Ageing Res Rev. 2024 Feb;94:102206. doi: 10.1016/j.arr.2024.102206. Epub 2024 Jan 24. Ageing Res Rev. 2024. PMID: 38278370 Review.
-
The role of Dipeptidyl Peptidase-4 in cutaneous disease.Exp Dermatol. 2021 Mar;30(3):304-318. doi: 10.1111/exd.14228. Epub 2020 Nov 19. Exp Dermatol. 2021. PMID: 33131073 Review.
Cited by
-
The Role of Senescence-Associated Secretory Phenotype in Bone Loss.Front Cell Dev Biol. 2022 Feb 9;10:841612. doi: 10.3389/fcell.2022.841612. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35223858 Free PMC article. Review.
-
PDK4 rescues high-glucose-induced senescent fibroblasts and promotes diabetic wound healing through enhancing glycolysis and regulating YAP and JNK pathway.Cell Death Discov. 2023 Nov 25;9(1):424. doi: 10.1038/s41420-023-01725-2. Cell Death Discov. 2023. PMID: 38001078 Free PMC article.
-
An IFI6-based hydrogel promotes the healing of radiation-induced skin injury through regulation of the HSF1 activity.J Nanobiotechnology. 2022 Jun 18;20(1):288. doi: 10.1186/s12951-022-01466-x. J Nanobiotechnology. 2022. PMID: 35717249 Free PMC article.
-
Development of a thiostrepton-free system for stable production of PLD in Streptomyces lividans SBT5.Microb Cell Fact. 2022 Dec 19;21(1):263. doi: 10.1186/s12934-022-01992-1. Microb Cell Fact. 2022. PMID: 36529749 Free PMC article.
-
Dipeptidylpeptidase-4-targeted activatable fluorescent probes visualize senescent cells.Cancer Sci. 2024 Aug;115(8):2762-2773. doi: 10.1111/cas.16229. Epub 2024 May 27. Cancer Sci. 2024. PMID: 38802068 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
