Cytosolic detection of phagosomal bacteria-Mechanisms underlying PAMP exodus from the phagosome into the cytosol
- PMID: 34738270
- PMCID: PMC8688326
- DOI: 10.1111/mmi.14841
Cytosolic detection of phagosomal bacteria-Mechanisms underlying PAMP exodus from the phagosome into the cytosol
Abstract
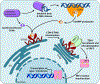
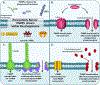
The metazoan innate immune system senses bacterial infections by detecting highly conserved bacterial molecules, termed pathogen-associated molecular patterns (PAMPs). PAMPs are detected by a variety of host pattern recognition receptors (PRRs), whose function is to coordinate downstream immune responses. PRR activities are, in part, regulated by their subcellular localizations. Accordingly, professional phagocytes can detect extracellular bacteria and their PAMPs via plasma membrane-oriented PRRs. Conversely, phagocytosed bacteria and their PAMPs are detected by transmembrane PRRs oriented toward the phagosomal lumen. Even though PAMPs are unable to passively diffuse across membranes, phagocytosed bacteria are also detected by PRRs localized within the host cell cytosol. This phenomenon is explained by phagocytosis of bacteria that specialize in phagosomal escape and cytosolic residence. Contrary to this cytosolic lifestyle, most bacteria studied to date spend their entire intracellular lifestyle contained within phagosomes, yet they also stimulate cytosolic PRRs. Herein, we will review our current understanding of how phagosomal PAMPs become accessible to cytosolic PRRs, as well as highlight knowledge gaps that should inspire future investigations.
Keywords: STING; cGAS; caspase-11; caspase-4; caspase-5; cyclic dinucleotides; guanylate binding proteins; lipopolysaccharide; macrophage; pathogen-associated molecular pattern; pattern recognition receptor; phagosome.
© 2021 John Wiley & Sons Ltd.
Figures

Similar articles
-
A method for functional trans-complementation of intracellular Francisella tularensis.PLoS One. 2014 Feb 4;9(2):e88194. doi: 10.1371/journal.pone.0088194. eCollection 2014. PLoS One. 2014. PMID: 24505427 Free PMC article.
-
Insights into phagocytosis-coupled activation of pattern recognition receptors and inflammasomes.Curr Opin Immunol. 2014 Feb;26:100-10. doi: 10.1016/j.coi.2013.11.003. Epub 2013 Dec 5. Curr Opin Immunol. 2014. PMID: 24556406 Free PMC article. Review.
-
Helicobacter pylori Pathogen-Associated Molecular Patterns: Friends or Foes?Int J Mol Sci. 2022 Mar 24;23(7):3531. doi: 10.3390/ijms23073531. Int J Mol Sci. 2022. PMID: 35408892 Free PMC article. Review.
-
Tumour viruses and innate immunity.Philos Trans R Soc Lond B Biol Sci. 2017 Oct 19;372(1732):20160267. doi: 10.1098/rstb.2016.0267. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28893934 Free PMC article. Review.
-
Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system.PLoS Pathog. 2007 Mar;3(3):e51. doi: 10.1371/journal.ppat.0030051. PLoS Pathog. 2007. PMID: 17397264 Free PMC article.
Cited by
-
Advances in stem cell therapy for peritoneal fibrosis: from mechanisms to therapeutics.Stem Cell Res Ther. 2023 Oct 10;14(1):293. doi: 10.1186/s13287-023-03520-3. Stem Cell Res Ther. 2023. PMID: 37817212 Free PMC article. Review.
-
The lncRNAs involved in regulating the RIG-I signaling pathway.Front Cell Infect Microbiol. 2022 Nov 9;12:1041682. doi: 10.3389/fcimb.2022.1041682. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36439216 Free PMC article. Review.
-
Scratching the Surface Takes a Toll: Immune Recognition of Viral Proteins by Surface Toll-like Receptors.Viruses. 2022 Dec 24;15(1):52. doi: 10.3390/v15010052. Viruses. 2022. PMID: 36680092 Free PMC article. Review.
-
LC3-Associated Phagocytosis in Bacterial Infection.Pathogens. 2022 Jul 30;11(8):863. doi: 10.3390/pathogens11080863. Pathogens. 2022. PMID: 36014984 Free PMC article. Review.
-
DNA-Dependent Interferon Induction and Lung Inflammation in Bordetella pertussis Infection.J Interferon Cytokine Res. 2023 Oct;43(10):478-486. doi: 10.1089/jir.2023.0066. Epub 2023 Aug 31. J Interferon Cytokine Res. 2023. PMID: 37651198 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

