Waning antibodies from inactivated SARS-CoV-2 vaccination offer protection against infection without antibody-enhanced immunopathology in rhesus macaque pneumonia models
- PMID: 34736354
- PMCID: PMC8635581
- DOI: 10.1080/22221751.2021.2002670
Waning antibodies from inactivated SARS-CoV-2 vaccination offer protection against infection without antibody-enhanced immunopathology in rhesus macaque pneumonia models
Abstract
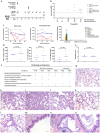
Inactivated coronaviruses, including severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and Middle East respiratory syndrome coronavirus (MERS-CoV), as potential vaccines have been reported to result in enhanced respiratory diseases (ERDs) in murine and nonhuman primate (NHP) pneumonia models after virus challenge, which poses great safety concerns of antibody-dependent enhancement (ADE) for the rapid wide application of inactivated SARS-CoV-2 vaccines in humans, especially when the neutralizing antibody levels induced by vaccination or initial infection quickly wane to nonneutralizing or subneutralizing levels over the time. With passive transfer of diluted postvaccination polyclonal antibodies to mimic the waning antibody responses after vaccination, we found that in the absence of cellular immunity, passive infusion of subneutralizing or nonneutralizing anti-SARS-CoV-2 antibodies could still provide some level of protection against infection upon challenge, and no low-level antibody-enhanced infection was observed. The anti-SARS-CoV-2 IgG-infused group and control group showed similar, mild to moderate pulmonary immunopathology during the acute phase of virus infection, and no evidence of vaccine-related pulmonary immunopathology enhancement was found. Typical immunopathology included elevated MCP-1, IL-8 and IL-33 in bronchoalveolar lavage fluid; alveolar epithelial hyperplasia; and exfoliated cells and mucus in bronchioles. Our results corresponded with the recent observations that no pulmonary immunology was detected in preclinical studies of inactivated SARS-CoV-2 vaccines in either murine or NHP pneumonia models or in large clinical trials and further supported the safety of inactivated SARS-CoV-2 vaccines.
Keywords: ADE; SARS-CoV-2; immunopathology; inactivated vaccines; subneutralizing antibodies.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
Similar articles
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Immune Profile and Clinical Outcome of Breakthrough Cases After Vaccination With an Inactivated SARS-CoV-2 Vaccine.Front Immunol. 2021 Sep 29;12:742914. doi: 10.3389/fimmu.2021.742914. eCollection 2021. Front Immunol. 2021. PMID: 34659237 Free PMC article. Clinical Trial.
-
A human cell-based SARS-CoV-2 vaccine elicits potent neutralizing antibody responses and protects mice from SARS-CoV-2 challenge.Emerg Microbes Infect. 2021 Dec;10(1):1555-1573. doi: 10.1080/22221751.2021.1957400. Emerg Microbes Infect. 2021. PMID: 34304724 Free PMC article.
-
Characterization of SARS-CoV-2-specific humoral immunity and its potential applications and therapeutic prospects.Cell Mol Immunol. 2022 Feb;19(2):150-157. doi: 10.1038/s41423-021-00774-w. Epub 2021 Oct 13. Cell Mol Immunol. 2022. PMID: 34645940 Free PMC article. Review.
-
Role of antibody-dependent enhancement (ADE) in the virulence of SARS-CoV-2 and its mitigation strategies for the development of vaccines and immunotherapies to counter COVID-19.Hum Vaccin Immunother. 2020 Dec 1;16(12):3055-3060. doi: 10.1080/21645515.2020.1796425. Epub 2020 Aug 26. Hum Vaccin Immunother. 2020. PMID: 32845733 Free PMC article. Review.
Cited by
-
Trends of humoral immune responses to heterologous antigenic exposure due to vaccination & omicron SARS-CoV-2 infection: Implications for boosting.Indian J Med Res. 2023 Jun;157(6):509-518. doi: 10.4103/ijmr.ijmr_2521_22. Indian J Med Res. 2023. PMID: 37322634 Free PMC article.
-
Role of the humoral immune response during COVID-19: guilty or not guilty?Mucosal Immunol. 2022 Jun;15(6):1170-1180. doi: 10.1038/s41385-022-00569-w. Epub 2022 Oct 4. Mucosal Immunol. 2022. PMID: 36195658 Free PMC article. Review.
-
In Vitro Antibody-Dependent Enhancement of SARS-CoV-2 Infection Could Be Abolished by Adding Human IgG.Pathogens. 2023 Aug 30;12(9):1108. doi: 10.3390/pathogens12091108. Pathogens. 2023. PMID: 37764916 Free PMC article.
-
Th2-Oriented Immune Serum After SARS-CoV-2 Vaccination Does Not Enhance Infection In Vitro.Front Immunol. 2022 Apr 8;13:882856. doi: 10.3389/fimmu.2022.882856. eCollection 2022. Front Immunol. 2022. PMID: 35464483 Free PMC article.
-
Profiles of Cough and Associated Risk Factors in Nonhospitalized Individuals With SARS-CoV-2 Omicron Variant Infection: Cross-Sectional Online Survey in China.JMIR Public Health Surveill. 2024 Feb 5;10:e47453. doi: 10.2196/47453. JMIR Public Health Surveill. 2024. PMID: 38315527 Free PMC article.
References
-
- Wang Q, Zhang L, Kuwahara K, et al. . Immunodominant SARS coronavirus epitopes in humans elicited both enhancing and neutralizing effects on infection in non-human primates. ACS Infect Dis. 2016 May 13;2(5):361–376. PubMed PMID: 27627203; PubMed Central PMCID: PMCPMC7075522. doi:10.1021/acsinfecdis.6b00006. - DOI - PMC - PubMed
-
- Agrawal AS, Tao X, Algaissi A, et al. . Immunization with inactivated Middle East respiratory syndrome coronavirus vaccine leads to lung immunopathology on challenge with live virus. Hum Vaccin Immunother. 2016 Sep;12(9):2351–2356. PubMed PMID: 27269431; PubMed Central PMCID: PMCPMC5027702. doi:10.1080/21645515.2016.1177688. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
