Arginine methylation and ubiquitylation crosstalk controls DNA end-resection and homologous recombination repair
- PMID: 34728620
- PMCID: PMC8564520
- DOI: 10.1038/s41467-021-26413-6
Arginine methylation and ubiquitylation crosstalk controls DNA end-resection and homologous recombination repair
Abstract
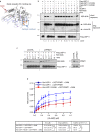
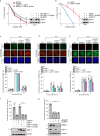
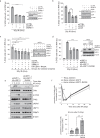
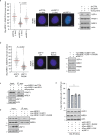
Cross-talk between distinct protein post-translational modifications is critical for an effective DNA damage response. Arginine methylation plays an important role in maintaining genome stability, but how this modification integrates with other enzymatic activities is largely unknown. Here, we identify the deubiquitylating enzyme USP11 as a previously uncharacterised PRMT1 substrate, and demonstrate that the methylation of USP11 promotes DNA end-resection and the repair of DNA double strand breaks (DSB) by homologous recombination (HR), an event that is independent from another USP11-HR activity, the deubiquitylation of PALB2. We also show that PRMT1 is a ubiquitylated protein that it is targeted for deubiquitylation by USP11, which regulates the ability of PRMT1 to bind to and methylate MRE11. Taken together, our findings reveal a specific role for USP11 during the early stages of DSB repair, which is mediated through its ability to regulate the activity of the PRMT1-MRE11 pathway.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
A glycine-arginine domain in control of the human MRE11 DNA repair protein.Mol Cell Biol. 2008 May;28(9):3058-69. doi: 10.1128/MCB.02025-07. Epub 2008 Feb 19. Mol Cell Biol. 2008. PMID: 18285453 Free PMC article.
-
A mouse PRMT1 null allele defines an essential role for arginine methylation in genome maintenance and cell proliferation.Mol Cell Biol. 2009 Jun;29(11):2982-96. doi: 10.1128/MCB.00042-09. Epub 2009 Mar 16. Mol Cell Biol. 2009. PMID: 19289494 Free PMC article.
-
GFI1 facilitates efficient DNA repair by regulating PRMT1 dependent methylation of MRE11 and 53BP1.Nat Commun. 2018 Apr 12;9(1):1418. doi: 10.1038/s41467-018-03817-5. Nat Commun. 2018. PMID: 29651020 Free PMC article.
-
Balancing act: To be, or not to be ubiquitylated.Mutat Res. 2017 Oct;803-805:43-50. doi: 10.1016/j.mrfmmm.2017.07.006. Epub 2017 Jul 21. Mutat Res. 2017. PMID: 28764946 Review.
-
Post-translational modification of factors involved in homologous recombination.DNA Repair (Amst). 2021 Aug;104:103114. doi: 10.1016/j.dnarep.2021.103114. Epub 2021 Jun 7. DNA Repair (Amst). 2021. PMID: 34111757 Review.
Cited by
-
GAPDH facilitates homologous recombination repair by stabilizing RAD51 in an HDAC1-dependent manner.EMBO Rep. 2023 Aug 3;24(8):e56437. doi: 10.15252/embr.202256437. Epub 2023 Jun 12. EMBO Rep. 2023. PMID: 37306047 Free PMC article.
-
Decoding Ubiquitin Modifications by Mass Spectrometry.Adv Exp Med Biol. 2024;1466:1-18. doi: 10.1007/978-981-97-7288-9_1. Adv Exp Med Biol. 2024. PMID: 39546132 Review.
-
Research progress on PRMTs involved in epigenetic modification and tumour signalling pathway regulation (Review).Int J Oncol. 2023 May;62(5):62. doi: 10.3892/ijo.2023.5510. Epub 2023 Apr 7. Int J Oncol. 2023. PMID: 37026519 Free PMC article. Review.
-
Cellular pathways influenced by protein arginine methylation: Implications for cancer.Mol Cell. 2021 Nov 4;81(21):4357-4368. doi: 10.1016/j.molcel.2021.09.011. Epub 2021 Oct 6. Mol Cell. 2021. PMID: 34619091 Free PMC article. Review.
-
Role of PRMT1 and PRMT5 in Breast Cancer.Int J Mol Sci. 2024 Aug 14;25(16):8854. doi: 10.3390/ijms25168854. Int J Mol Sci. 2024. PMID: 39201539 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

