Interplay between ADP-ribosyltransferases and essential cell signaling pathways controls cellular responses
- PMID: 34725336
- PMCID: PMC8560908
- DOI: 10.1038/s41421-021-00323-9
Interplay between ADP-ribosyltransferases and essential cell signaling pathways controls cellular responses
Abstract
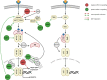
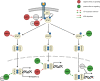
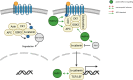
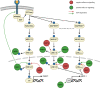
Signaling cascades provide integrative and interactive frameworks that allow the cell to respond to signals from its environment and/or from within the cell itself. The dynamic regulation of mammalian cell signaling pathways is often modulated by cascades of protein post-translational modifications (PTMs). ADP-ribosylation is a PTM that is catalyzed by ADP-ribosyltransferases and manifests as mono- (MARylation) or poly- (PARylation) ADP-ribosylation depending on the addition of one or multiple ADP-ribose units to protein substrates. ADP-ribosylation has recently emerged as an important cell regulator that impacts a plethora of cellular processes, including many intracellular signaling events. Here, we provide an overview of the interplay between the intracellular diphtheria toxin-like ADP-ribosyltransferase (ARTD) family members and five selected signaling pathways (including NF-κB, JAK/STAT, Wnt-β-catenin, MAPK, PI3K/AKT), which are frequently described to control or to be controlled by ADP-ribosyltransferases and how these interactions impact the cellular responses.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
A role of intracellular mono-ADP-ribosylation in cancer biology.FEBS J. 2013 Aug;280(15):3551-62. doi: 10.1111/febs.12290. Epub 2013 May 10. FEBS J. 2013. PMID: 23590234 Review.
-
ADP-Ribosylation Post-Translational Modification: An Overview with a Focus on RNA Biology and New Pharmacological Perspectives.Biomolecules. 2022 Mar 13;12(3):443. doi: 10.3390/biom12030443. Biomolecules. 2022. PMID: 35327636 Free PMC article. Review.
-
Intracellular Mono-ADP-Ribosylation in Signaling and Disease.Cells. 2015 Sep 25;4(4):569-95. doi: 10.3390/cells4040569. Cells. 2015. PMID: 26426055 Free PMC article. Review.
-
Players in ADP-ribosylation: Readers and Erasers.Curr Protein Pept Sci. 2016;17(7):654-667. doi: 10.2174/1389203717666160419144846. Curr Protein Pept Sci. 2016. PMID: 27090904 Review.
-
Towards small molecule inhibitors of mono-ADP-ribosyltransferases.Eur J Med Chem. 2015 May 5;95:546-51. doi: 10.1016/j.ejmech.2015.03.067. Epub 2015 Apr 1. Eur J Med Chem. 2015. PMID: 25847771
Cited by
-
Identification of porcine PARP11 as a restricted factor for pseudorabies virus.Front Cell Infect Microbiol. 2024 Oct 9;14:1414827. doi: 10.3389/fcimb.2024.1414827. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39445214 Free PMC article.
-
Poly(ADP-Ribose) Polymerase-1 Regulates Pyroptosis Independent Function of NLRP3 Inflammasome in Neutrophil Extracellular Trap Formation.Immunohorizons. 2024 Aug 1;8(8):586-597. doi: 10.4049/immunohorizons.2400058. Immunohorizons. 2024. PMID: 39186692 Free PMC article.
-
14-3-3 Activated Bacterial Exotoxins AexT and ExoT Share Actin and the SH2 Domains of CRK Proteins as Targets for ADP-Ribosylation.Pathogens. 2022 Dec 8;11(12):1497. doi: 10.3390/pathogens11121497. Pathogens. 2022. PMID: 36558830 Free PMC article.
-
A Combined Gas-Phase Separation Strategy for ADP-ribosylated Peptides.J Am Soc Mass Spectrom. 2023 Oct 4;34(10):2136-2145. doi: 10.1021/jasms.3c00129. Epub 2023 Aug 17. J Am Soc Mass Spectrom. 2023. PMID: 37589412 Free PMC article.
-
Proteostasis and neurodegeneration: a closer look at autophagy in Alzheimer's disease.Front Aging Neurosci. 2023 Nov 2;15:1281338. doi: 10.3389/fnagi.2023.1281338. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38020769 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

