Recent advances in nucleotide analogue-based techniques for tracking dividing stem cells: An overview
- PMID: 34717955
- PMCID: PMC8592869
- DOI: 10.1016/j.jbc.2021.101345
Recent advances in nucleotide analogue-based techniques for tracking dividing stem cells: An overview
Abstract
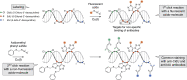
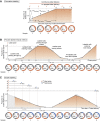
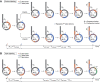
Detection of thymidine analogues after their incorporation into replicating DNA represents a powerful tool for the study of cellular DNA synthesis, progression through the cell cycle, cell proliferation kinetics, chronology of cell division, and cell fate determination. Recent advances in the concurrent detection of multiple such analogues offer new avenues for the investigation of unknown features of these vital cellular processes. Combined with quantitative analysis, temporal discrimination of multiple labels enables elucidation of various aspects of stem cell life cycle in situ, such as division modes, differentiation, maintenance, and elimination. Data obtained from such experiments are critically important for creating descriptive models of tissue histogenesis and renewal in embryonic development and adult life. Despite the wide use of thymidine analogues in stem cell research, there are a number of caveats to consider for obtaining valid and reliable labeling results when marking replicating DNA with nucleotide analogues. Therefore, in this review, we describe critical points regarding dosage, delivery, and detection of nucleotide analogues in the context of single and multiple labeling, outline labeling schemes based on pulse-chase, cumulative and multilabel marking of replicating DNA for revealing stem cell proliferative behaviors, and determining cell cycle parameters, and discuss preconditions and pitfalls in conducting such experiments. The information presented in our review is important for rational design of experiments on tracking dividing stem cells by marking replicating DNA with thymidine analogues.
Keywords: BrdU (5-bromo-2′-deoxyuridine); EdU (5-ethynyl-2′-deoxyuridine); click chemistry; immunohistochemistry; proliferation; stem cells; thymidine analogues.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures

Similar articles
-
Methods for Inferring Cell Cycle Parameters Using Thymidine Analogues.Biology (Basel). 2023 Jun 20;12(6):885. doi: 10.3390/biology12060885. Biology (Basel). 2023. PMID: 37372169 Free PMC article. Review.
-
The bioavailability time of commonly used thymidine analogues after intraperitoneal delivery in mice: labeling kinetics in vivo and clearance from blood serum.Histochem Cell Biol. 2022 Feb;157(2):239-250. doi: 10.1007/s00418-021-02048-y. Epub 2021 Nov 10. Histochem Cell Biol. 2022. PMID: 34757474 Free PMC article.
-
Thymidine analogues for tracking DNA synthesis.Molecules. 2011 Sep 15;16(9):7980-93. doi: 10.3390/molecules16097980. Molecules. 2011. PMID: 21921870 Free PMC article. Review.
-
Cell-cycle analyses using thymidine analogues in fission yeast.PLoS One. 2014 Feb 13;9(2):e88629. doi: 10.1371/journal.pone.0088629. eCollection 2014. PLoS One. 2014. PMID: 24551125 Free PMC article.
-
Triple S-Phase Labeling of Dividing Stem Cells.Stem Cell Reports. 2018 Feb 13;10(2):615-626. doi: 10.1016/j.stemcr.2017.12.020. Stem Cell Reports. 2018. PMID: 29358087 Free PMC article.
Cited by
-
Synthetic Thymidine Analog Labeling without Misconceptions.Cells. 2022 Jun 10;11(12):1888. doi: 10.3390/cells11121888. Cells. 2022. PMID: 35741018 Free PMC article.
-
Methods for Inferring Cell Cycle Parameters Using Thymidine Analogues.Biology (Basel). 2023 Jun 20;12(6):885. doi: 10.3390/biology12060885. Biology (Basel). 2023. PMID: 37372169 Free PMC article. Review.
-
Tracking cell-type-specific temporal dynamics in human and mouse brains.Cell. 2023 Sep 28;186(20):4345-4364.e24. doi: 10.1016/j.cell.2023.08.042. Cell. 2023. PMID: 37774676 Free PMC article.
-
Nucleotide excision repair removes thymidine analog 5-ethynyl-2'-deoxyuridine from the mammalian genome.Proc Natl Acad Sci U S A. 2022 Aug 30;119(35):e2210176119. doi: 10.1073/pnas.2210176119. Epub 2022 Aug 22. Proc Natl Acad Sci U S A. 2022. PMID: 35994676 Free PMC article.
-
Actively replicating gut bacteria identified by 5-ethynyl-2'-deoxyuridine (EdU) click chemistry and cell sorting.Gut Microbes. 2023 Jan-Dec;15(1):2180317. doi: 10.1080/19490976.2023.2180317. Gut Microbes. 2023. PMID: 36823031 Free PMC article.
References
-
- Taupin P. Nova Biomedical, Nova Science Publishers; New York, NY: 2008. Stem Cells and Regenerative Medicine.
-
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: Paradigms, pitfalls, limitations, and validation. Brain Res. Rev. 2007;53:198–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

