The Mucosal and Serological Immune Responses to the Novel Coronavirus (SARS-CoV-2) Vaccines
- PMID: 34712232
- PMCID: PMC8547269
- DOI: 10.3389/fimmu.2021.744887
The Mucosal and Serological Immune Responses to the Novel Coronavirus (SARS-CoV-2) Vaccines
Abstract
Background: Although the serological antibody responses induced by SARS-CoV-2 vaccines are well characterized, little is known about their ability to elicit mucosal immunity.
Objectives: This study aims to examine and compare the mucosal and systemic responses of recipients of two different vaccination platforms: mRNA (Comirnaty) and inactivated virus (CoronaVac).
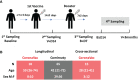
Methods: Serial blood and nasal epithelial lining fluid (NELF) samples were collected from the recipients of either Comirnaty or CoronaVac. The plasma and NELF immunoglobulins A and G (IgA and IgG) specific to SARS-CoV-2 S1 protein (S1) and their neutralization effects were quantified.
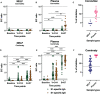
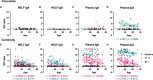
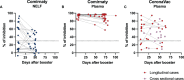
Results: Comirnaty induced nasal S1-specific immunoglobulin responses, which were evident as early as 14 ± 2 days after the first dose. In 64% of the subjects, the neutralizing effects of NELF persisted for at least 50 days. Moreover, 85% of Comirnaty recipients exhibited S1-specific IgA and IgG responses in plasma by 14 ± 2 days after the first dose. By 7 ± 2 days after the booster, all plasma samples possessed S1-specific IgA and IgG responses and were neutralizing. The induction of S1-specific plasma antibodies by CoronaVac was IgG dominant, and 83% of the subjects possessed S1-specific IgG by 7 ± 2 days after the booster, with neutralizing effects.
Conclusion: Comirnaty induces S1-specific IgA and IgG responses with neutralizing activity in the nasal mucosa; a similar response is not seen with CoronaVac.
Clinical implication: The presence of a nasal response with mRNA vaccine may provide additional protection compared with inactivated virus vaccine. However, whether such widespread immunological response may produce inadvertent adverse effects in other tissues warrants further investigation.
Keywords: SARS-CoV-2; immunoglobulin A; immunoglobulin G; inactivated virus vaccine; mRNA vaccine; mucosal immunity; nasal epithelial lining fluid; serological immunity.
Copyright © 2021 Chan, Liu, Cheung, Tsun, Chan, Chan, Fung, Li and Lam.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Mucosal Vaccines, Sterilizing Immunity, and the Future of SARS-CoV-2 Virulence.Viruses. 2022 Jan 19;14(2):187. doi: 10.3390/v14020187. Viruses. 2022. PMID: 35215783 Free PMC article. Review.
-
Response and Duration of Serum Anti-SARS-CoV-2 Antibodies After Inactivated Vaccination Within 160 Days.Front Immunol. 2021 Dec 23;12:786554. doi: 10.3389/fimmu.2021.786554. eCollection 2021. Front Immunol. 2021. PMID: 35003104 Free PMC article.
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
SARS-CoV-2 specific antibody responses after third CoronaVac or BNT162b2 vaccine following two-dose CoronaVac vaccine regimen.J Med Virol. 2022 Jan;94(1):39-41. doi: 10.1002/jmv.27350. Epub 2021 Sep 21. J Med Virol. 2022. PMID: 34536028 No abstract available.
-
A Review of the Progress and Challenges of Developing a Vaccine for COVID-19.Front Immunol. 2020 Oct 14;11:585354. doi: 10.3389/fimmu.2020.585354. eCollection 2020. Front Immunol. 2020. PMID: 33163000 Free PMC article. Review.
Cited by
-
A comprehensive review of BBV152 vaccine development, effectiveness, safety, challenges, and prospects.Front Immunol. 2022 Sep 13;13:940715. doi: 10.3389/fimmu.2022.940715. eCollection 2022. Front Immunol. 2022. PMID: 36177016 Free PMC article. Review.
-
Differentially induced immunity in buccal and nasal mucosae after vaccination for SARS-CoV-2: Prospects for mass scale immunity-screening in large populations.Front Immunol. 2022 Nov 17;13:999693. doi: 10.3389/fimmu.2022.999693. eCollection 2022. Front Immunol. 2022. PMID: 36466833 Free PMC article.
-
Induction of systemic, mucosal, and cellular immunity against SARS-CoV-2 in mice vaccinated by trans-airway with a S1 protein combined with a pulmonary surfactant-derived adjuvant SF-10.Influenza Other Respir Viruses. 2023 Mar 9;17(3):e13119. doi: 10.1111/irv.13119. eCollection 2023 Mar. Influenza Other Respir Viruses. 2023. PMID: 36909295 Free PMC article.
-
Mucosal Vaccines, Sterilizing Immunity, and the Future of SARS-CoV-2 Virulence.Viruses. 2022 Jan 19;14(2):187. doi: 10.3390/v14020187. Viruses. 2022. PMID: 35215783 Free PMC article. Review.
-
Durability of immune responses to SARS-CoV-2 infection and vaccination.Semin Immunol. 2024 May;73:101884. doi: 10.1016/j.smim.2024.101884. Epub 2024 Jun 10. Semin Immunol. 2024. PMID: 38861769 Review.
References
-
- Organization WH . WHO Coronavirus (COVID-19) Dashboard. (2021). WHO Press - World Health Organization.
-
- International Nonproprietary Names Programme . Messenger RNA Encoding the Full-Length SARS-CoV-2 Spike Glycoprotein. World Health Organization; (2020). WHO Press - World Health Organization.
-
- Wu Z, Hu Y, Xu M, Chen Z, Yang W, Jiang Z, et al. . Safety, Tolerability, and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine (CoronaVac) in Healthy Adults Aged 60 Years and Older: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Clinical Trial. Lancet Infect Dis (2021) 21(6):803–12. doi: 10.1016/S1473-3099(20)30987-7 - DOI - PMC - PubMed
-
- Zhang Y, Zeng G, Pan H, Li C, Hu Y, Chu K, et al. . Safety, Tolerability, and Immunogenicity of an Inactivated SARS-CoV-2 Vaccine in Healthy Adults Aged 18-59 Years: A Randomised, Double-Blind, Placebo-Controlled, Phase 1/2 Clinical Trial. Lancet Infect Dis (2021) 21(2):181–92. doi: 10.1016/S1473-3099(20)30843-4 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

