Non-Stimulatory pMHC Enhance CD8 T Cell Effector Functions by Recruiting Coreceptor-Bound Lck
- PMID: 34707605
- PMCID: PMC8542885
- DOI: 10.3389/fimmu.2021.721722
Non-Stimulatory pMHC Enhance CD8 T Cell Effector Functions by Recruiting Coreceptor-Bound Lck
Abstract
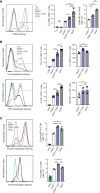
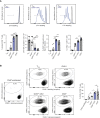
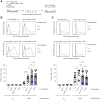
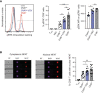
Under physiological conditions, CD8+ T cells need to recognize low numbers of antigenic pMHC class I complexes in the presence of a surplus of non-stimulatory, self pMHC class I on the surface of the APC. Non-stimulatory pMHC have been shown to enhance CD8+ T cell responses to low amounts of antigenic pMHC, in a phenomenon called co-agonism, but the physiological significance and molecular mechanism of this phenomenon are still poorly understood. Our data show that co-agonist pMHC class I complexes recruit CD8-bound Lck to the immune synapse to modulate CD8+ T cell signaling pathways, resulting in enhanced CD8+ T cell effector functions and proliferation, both in vitro and in vivo. Moreover, co-agonism can boost T cell proliferation through an extrinsic mechanism, with co-agonism primed CD8+ T cells enhancing Akt pathway activation and proliferation in neighboring CD8+ T cells primed with low amounts of antigen.
Keywords: AKT pathway; Lck; T cell effector functions; T cell receptor; T cell signaling; co-agonism; non-stimulatory peptide MHC.
Copyright © 2021 Zhao, Wu, Ng, Leow, Wei, Gascoigne and Brzostek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
CD4+ Th-APC with acquired peptide/MHC class I and II complexes stimulate type 1 helper CD4+ and central memory CD8+ T cell responses.J Immunol. 2009 Jan 1;182(1):193-206. doi: 10.4049/jimmunol.182.1.193. J Immunol. 2009. PMID: 19109150
-
CD4 and CD8 binding to MHC molecules primarily acts to enhance Lck delivery.Proc Natl Acad Sci U S A. 2010 Sep 28;107(39):16916-21. doi: 10.1073/pnas.1010568107. Epub 2010 Sep 13. Proc Natl Acad Sci U S A. 2010. PMID: 20837541 Free PMC article.
-
Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse.Nature. 2014 Mar 6;507(7490):118-23. doi: 10.1038/nature12951. Epub 2014 Feb 2. Nature. 2014. PMID: 24487619 Free PMC article.
-
Spatial coordination of CD8 and TCR molecules controls antigen recognition by CD8+ T-cells.FEBS Lett. 2005 Jun 13;579(15):3336-41. doi: 10.1016/j.febslet.2005.04.025. Epub 2005 Apr 26. FEBS Lett. 2005. PMID: 15913613 Review.
-
The roles of CD4 and CD8 in T cell activation.Semin Immunol. 1991 May;3(3):133-41. Semin Immunol. 1991. PMID: 1909592 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

