TRPC6, a therapeutic target for pulmonary hypertension
- PMID: 34704831
- PMCID: PMC8715021
- DOI: 10.1152/ajplung.00159.2021
TRPC6, a therapeutic target for pulmonary hypertension
Abstract
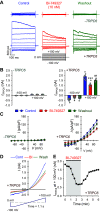
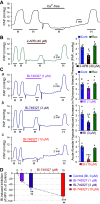
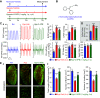
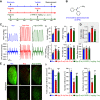
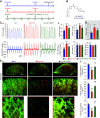
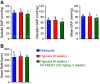
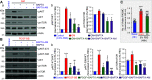
Idiopathic pulmonary arterial hypertension (PAH) is a fatal and progressive disease. Sustained vasoconstriction due to pulmonary arterial smooth muscle cell (PASMC) contraction and concentric arterial remodeling due partially to PASMC proliferation are the major causes for increased pulmonary vascular resistance and increased pulmonary arterial pressure in patients with precapillary pulmonary hypertension (PH) including PAH and PH due to respiratory diseases or hypoxemia. We and others observed upregulation of TRPC6 channels in PASMCs from patients with PAH. A rise in cytosolic Ca2+ concentration ([Ca2+]cyt) in PASMC triggers PASMC contraction and vasoconstriction, while Ca2+-dependent activation of PI3K/AKT/mTOR pathway is a pivotal signaling cascade for cell proliferation and gene expression. Despite evidence supporting a pathological role of TRPC6, no selective and orally bioavailable TRPC6 antagonist has yet been developed and tested for treatment of PAH or PH. In this study, we sought to investigate whether block of receptor-operated Ca2+ channels using a nonselective blocker of cation channels, 2-aminoethyl diphenylborinate (2-APB, administered intraperitoneally) and a selective blocker of TRPC6, BI-749327 (administered orally) can reverse established PH in mice. The results from the study show that intrapulmonary application of 2-APB (40 µM) or BI-749327 (3-10 µM) significantly and reversibly inhibited acute alveolar hypoxia-induced pulmonary vasoconstriction. Intraperitoneal injection of 2-APB (1 mg/kg per day) significantly attenuated the development of PH and partially reversed established PH in mice. Oral gavage of BI-749327 (30 mg/kg, every day, for 2 wk) reversed established PH by ∼50% via regression of pulmonary vascular remodeling. Furthermore, 2-APB and BI-749327 both significantly inhibited PDGF- and serum-mediated phosphorylation of AKT and mTOR in PASMC. In summary, the receptor-operated and mechanosensitive TRPC6 channel is a good target for developing novel treatment for PAH/PH. BI-749327, a selective TRPC6 blocker, is potentially a novel and effective drug for treating PAH and PH due to respiratory diseases or hypoxemia.
Keywords: BI-749327; calcium signaling; hypoxic pulmonary vasoconstriction; pulmonary hypertension; transient receptor potential channel.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Loss of acid-sensing ion channel 2 enhances pulmonary vascular resistance and hypoxic pulmonary hypertension.J Appl Physiol (1985). 2019 Aug 1;127(2):393-407. doi: 10.1152/japplphysiol.00894.2018. Epub 2019 Jun 6. J Appl Physiol (1985). 2019. PMID: 31169471 Free PMC article.
-
5-Aminoimidazole-4-carboxamide ribonucleotide formyltransferase/inosine monophosphate cyclohydrolase promotes pulmonary arterial smooth muscle cell proliferation via the Ras signaling pathway.Am J Physiol Cell Physiol. 2024 Oct 1;327(4):C901-C912. doi: 10.1152/ajpcell.00262.2024. Epub 2024 Aug 12. Am J Physiol Cell Physiol. 2024. PMID: 39129491
-
lncRNA VELRP Modulates Pulmonary Arterial Smooth Muscle Cell Proliferation and Promotes Vascular Remodeling in Pulmonary Hypertension.Arterioscler Thromb Vasc Biol. 2024 Dec;44(12):2560-2576. doi: 10.1161/ATVBAHA.124.321416. Epub 2024 Oct 3. Arterioscler Thromb Vasc Biol. 2024. PMID: 39360410
-
Guanylate cyclase stimulators for pulmonary hypertension.Cochrane Database Syst Rev. 2016 Aug 2;2016(8):CD011205. doi: 10.1002/14651858.CD011205.pub2. Cochrane Database Syst Rev. 2016. PMID: 27482837 Free PMC article. Review.
-
Oral budesonide for induction of remission in ulcerative colitis.Cochrane Database Syst Rev. 2015 Oct 26;2015(10):CD007698. doi: 10.1002/14651858.CD007698.pub3. Cochrane Database Syst Rev. 2015. PMID: 26497719 Free PMC article. Review.
Cited by
-
Transient Receptor Potential (TRP) Channels in Airway Toxicity and Disease: An Update.Cells. 2022 Sep 17;11(18):2907. doi: 10.3390/cells11182907. Cells. 2022. PMID: 36139480 Free PMC article. Review.
-
Bioinformatics analysis to identify potential biomarkers for the pulmonary artery hypertension associated with the basement membrane.Open Life Sci. 2023 Sep 26;18(1):20220730. doi: 10.1515/biol-2022-0730. eCollection 2023. Open Life Sci. 2023. PMID: 37772261 Free PMC article.
-
METTL3 Promotes Endothelium-Mesenchymal Transition of Pulmonary Artery Endothelial Cells by Regulating TRPC6/Calcineurin/NFAT Signaling Pathways.Evid Based Complement Alternat Med. 2023 Feb 21;2023:8269356. doi: 10.1155/2023/8269356. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 36865750 Free PMC article.
-
Role of Store-Operated Ca2+ Entry in the Pulmonary Vascular Remodeling Occurring in Pulmonary Arterial Hypertension.Biomolecules. 2021 Nov 27;11(12):1781. doi: 10.3390/biom11121781. Biomolecules. 2021. PMID: 34944425 Free PMC article. Review.
-
A Pharmacokinetic and Metabolism Study of the TRPC6 Inhibitor SH045 in Mice by LC-MS/MS.Int J Mol Sci. 2022 Mar 26;23(7):3635. doi: 10.3390/ijms23073635. Int J Mol Sci. 2022. PMID: 35408998 Free PMC article.
References
-
- Frost AE, Badesch DB, Barst RJ, Benza RL, Elliott CG, Farber HW, Krichman A, Liou TG, Raskob GE, Wason P, Feldkircher K, Turner M, McGoon MD. The changing picture of patients with pulmonary arterial hypertension in the United States: how REVEAL differs from historic and non-US Contemporary Registries. Chest 139: 128–137, 2011. doi:10.1378/chest.10-0075. - DOI - PubMed
-
- Hassoun PM, Mouthon L, Barbera JA, Eddahibi S, Flores SC, Grimminger F, Jones PL, Maitland ML, Michelakis ED, Morrell NW, Newman JH, Rabinovitch M, Schermuly R, Stenmark KR, Voelkel NF, Yuan JX, Humbert M. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 54: S10–S19, 2009. doi:10.1016/j.jacc.2009.04.006. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

