Andrographolide and Its Derivative Potassium Dehydrographolide Succinate Suppress PRRSV Replication in Primary and Established Cells via Differential Mechanisms of Action
- PMID: 34704222
- PMCID: PMC8692552
- DOI: 10.1007/s12250-021-00455-y
Andrographolide and Its Derivative Potassium Dehydrographolide Succinate Suppress PRRSV Replication in Primary and Established Cells via Differential Mechanisms of Action
Abstract
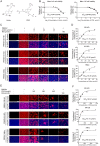
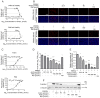
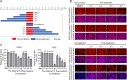
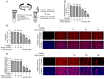
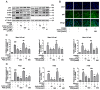
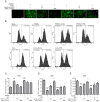
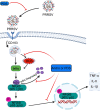
Porcine reproductive and respiratory syndrome virus (PRRSV) continues to cause significant economic loss worldwide and remains a serious threat to the pork industry. Currently, vaccination strategies provide limited protection against PRRSV infection, and consequently, new antiviral strategies are urgently required. Andrographolide (Andro) and its derivative potassium dehydrographolide succinate (PDS) have been used clinically in China and other Asian countries as therapies for inflammation-related diseases, including bacterial and viral infections, for decades. Here, we demonstrate that Andro and PDS exhibit robust activity against PRRSV replication in Marc-145 cells and primary porcine alveolar macrophages (PAMs). The two compounds exhibited broad-spectrum inhibitory activities in vitro against clinically circulating type 2 PRRSV GD-HD, XH-GD, and NADC30-like HNhx strains in China. The EC50 values of Andro against three tested PRRSV strain infections in Marc-145 cells ranged from 11.7 to 15.3 μmol/L, with selectivity indexes ranging from 8.3 to 10.8, while the EC50 values of PDS ranged from 57.1 to 85.4 μmol/L, with selectivity indexes ranging from 344 to 515. Mechanistically, the anti-PRRSV activity of the two compounds is closely associated with their potent suppression on NF-κB activation and enhanced oxidative stress induced by PRRSV infection. Further mechanistic investigations revealed that PDS, but not Andro, is able to directly interact with PRRSV particles. Taken together, our findings suggest that Andro and PDS are promising PRRSV inhibitors in vitro and deserves further in vivo studies in swine.
Keywords: Andrographolide (Andro); Inhibit; NF-κB signaling pathway; Oxidative stress; Porcine reproductive and respiratory syndrome virus (PRRSV); Potassium dehydrographolide succinate (PDS).
© 2021. Wuhan Institute of Virology, CAS.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


Similar articles
-
Platycodin D Suppresses Type 2 Porcine Reproductive and Respiratory Syndrome Virus In Primary and Established Cell Lines.Viruses. 2018 Nov 21;10(11):657. doi: 10.3390/v10110657. Viruses. 2018. PMID: 30469357 Free PMC article.
-
Inhibition of proanthocyanidin A2 on porcine reproductive and respiratory syndrome virus replication in vitro.PLoS One. 2018 Feb 28;13(2):e0193309. doi: 10.1371/journal.pone.0193309. eCollection 2018. PLoS One. 2018. PMID: 29489892 Free PMC article.
-
The Antimalaria Drug Artesunate Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication by Activating AMPK and Nrf2/HO-1 Signaling Pathways.J Virol. 2022 Feb 9;96(3):e0148721. doi: 10.1128/JVI.01487-21. Epub 2021 Nov 17. J Virol. 2022. PMID: 34787456 Free PMC article.
-
Ginsenoside Rg1 Suppresses Type 2 PRRSV Infection via NF-κB Signaling Pathway In Vitro, and Provides Partial Protection against HP-PRRSV in Piglet.Viruses. 2019 Nov 10;11(11):1045. doi: 10.3390/v11111045. Viruses. 2019. PMID: 31717616 Free PMC article.
-
Ursolic acid derivatives are potent inhibitors against porcine reproductive and respiratory syndrome virus.RSC Adv. 2020 Jun 15;10(38):22783-22796. doi: 10.1039/d0ra04070c. eCollection 2020 Jun 10. RSC Adv. 2020. PMID: 35514592 Free PMC article.
Cited by
-
Toosendanin suppresses African swine fever virus replication through upregulating interferon regulatory factor 1 in porcine alveolar macrophage cultures.Front Microbiol. 2022 Aug 30;13:970501. doi: 10.3389/fmicb.2022.970501. eCollection 2022. Front Microbiol. 2022. PMID: 36110293 Free PMC article.
-
Betulonic Acid Inhibits Type-2 Porcine Reproductive and Respiratory Syndrome Virus Replication by Downregulating Cellular ATP Production.Int J Mol Sci. 2024 Sep 26;25(19):10366. doi: 10.3390/ijms251910366. Int J Mol Sci. 2024. PMID: 39408695 Free PMC article.
-
Integrating artificial intelligence into the modernization of traditional Chinese medicine industry: a review.Front Pharmacol. 2024 Feb 23;15:1181183. doi: 10.3389/fphar.2024.1181183. eCollection 2024. Front Pharmacol. 2024. PMID: 38464717 Free PMC article. Review.
-
Potassium Dehydroandrograpolide Succinate Targets NRP1 Mediated VEGFR2/VE-Cadherin Signaling Pathway to Promote Endothelial Barrier Repair.Int J Mol Sci. 2023 Feb 4;24(4):3096. doi: 10.3390/ijms24043096. Int J Mol Sci. 2023. PMID: 36834519 Free PMC article.
References
-
- Ait-Ali T, Wilson AD, Westcott DG, Clapperton M, Waterfall M, Mellencamp MA, Drew TW, Bishop SC, Archibald AL. Innate immune responses to replication of porcine reproductive and respiratory syndrome virus in isolated Swine alveolar macrophages. Viral Immunol. 2007;20:105–118. - PubMed
-
- Asamitsu K, Yamaguchi T, Nakata K, Hibi Y, Victoriano AF, Imai K, Onozaki K, Kitade Y. Inhibition of human immunodeficiency virus type 1 replication by blocking IkappaB kinase with noraristeromycin. J Biochem. 2008;144:581–589. - PubMed
-
- Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. - PubMed
-
- Cáceres DD, Hancke JL, Burgos RA, Sandberg F, Wikman GK. Use of visual analogue scale measurements (VAS) to asses the effectiveness of standardized Andrographis paniculata extract SHA-10 in reducing the symptoms of common cold. A randomized double blind-placebo study. Phytomedicine. 1999;6:217–223. - PubMed
-
- Calabrese C, Berman SH, Babish JG, Ma X, Shinto L, Dorr M, Wells K, Wenner CA, Standish LJ. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytother Res. 2000;14:333–338. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

