Can Soluble Immune Checkpoint Molecules on Exosomes Mediate Inflammation?
- PMID: 34697721
- PMCID: PMC10128092
- DOI: 10.1007/s11481-021-10018-3
Can Soluble Immune Checkpoint Molecules on Exosomes Mediate Inflammation?
Abstract
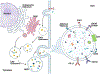
Immune checkpoints (ICPs) are major co-signaling pathways that trigger effector functions in immune cells, with isoforms that are either membrane bound, engaging in direct cell to cell activation locally, or soluble, acting at distant sites by circulating freely or potentially via extracellular vesicles (EVs). Exosomes are small EVs secreted by a variety of cells carrying various proteins and nucleic acids. They are distributed extensively through biological fluids and have major impacts on infectious diseases, cancer, and neuroinflammation. Similarly, ICPs play key roles in a variety of disease conditions and have been extensively utilized as a prognostic tool for various cancers. Herein, we explored if the association between exosomes and ICPs could be a significant contributor of inflammation, particularly in the setting of cancer, neuroinflammation and viral infections, wherein the up regulation in both exosomal proteins and ICPs correlate with immunosuppressive effects. The detailed literature review of existing data highlights the significance and complexity of these two important pathways in mediating cancer and potentiating neuroinflammation via modulating overall immune response. Cells increasingly secret exosomes in response to intracellular signals from invading pathogens or cancerous transformations. These exosomes can carry a variety of cargo including proteins, nucleic acids, cytokines, and receptors/ligands that have functional consequences on recipient cells. Illustration generated using BioRender software.
Keywords: Cancer; Exosome; Immune checkpoints; Neuroinflammation; Viral infections.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Figures

Similar articles
-
Extracellular Vesicles in Oncology: from Immune Suppression to Immunotherapy.AAPS J. 2021 Feb 14;23(2):30. doi: 10.1208/s12248-021-00554-4. AAPS J. 2021. PMID: 33586060 Free PMC article. Review.
-
The role of extracellular vesicle immune checkpoints in cancer.Clin Exp Immunol. 2024 May 16;216(3):230-239. doi: 10.1093/cei/uxae026. Clin Exp Immunol. 2024. PMID: 38518192 Review.
-
A Protocol for Isolation, Purification, Characterization, and Functional Dissection of Exosomes.Methods Mol Biol. 2021;2261:105-149. doi: 10.1007/978-1-0716-1186-9_9. Methods Mol Biol. 2021. PMID: 33420988
-
Exosome and Secretion: Action On?Adv Exp Med Biol. 2020;1248:455-483. doi: 10.1007/978-981-15-3266-5_19. Adv Exp Med Biol. 2020. PMID: 32185722 Review.
-
Functional significance of macrophage-derived exosomes in inflammation and pain.Pain. 2014 Aug;155(8):1527-1539. doi: 10.1016/j.pain.2014.04.029. Epub 2014 Apr 30. Pain. 2014. PMID: 24792623 Free PMC article.
Cited by
-
Antibody and Cell-Based Therapies against Virus-Induced Cancers in the Context of HIV/AIDS.Pathogens. 2023 Dec 22;13(1):14. doi: 10.3390/pathogens13010014. Pathogens. 2023. PMID: 38251321 Free PMC article. Review.
-
Soluble immune checkpoints: implications for cancer prognosis and response to immune checkpoint therapy and conventional therapies.J Exp Clin Cancer Res. 2024 May 31;43(1):155. doi: 10.1186/s13046-024-03074-z. J Exp Clin Cancer Res. 2024. PMID: 38822401 Free PMC article. Review.
-
Prognostic impacts of soluble immune checkpoint regulators and cytokines in patients with SARS-CoV-2 infection.Front Immunol. 2022 Aug 15;13:903419. doi: 10.3389/fimmu.2022.903419. eCollection 2022. Front Immunol. 2022. PMID: 36045684 Free PMC article.
-
Retroviral b-Zip protein (HBZ) contributes to the release of soluble and exosomal immune checkpoint molecules in the context of neuroinflammation.J Extracell Biol. 2023 Jul;2(7):e102. doi: 10.1002/jex2.102. Epub 2023 Jul 17. J Extracell Biol. 2023. PMID: 37547182 Free PMC article.
-
Exploring the potential of the convergence between extracellular vesicles and CAR technology as a novel immunotherapy approach.J Extracell Biol. 2024 Sep 26;3(9):e70011. doi: 10.1002/jex2.70011. eCollection 2024 Sep. J Extracell Biol. 2024. PMID: 39328262 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

